Metals and Acids expsPage
1
1
Slide 1
METALS AND ACIDS
Slide 2
Metals and ACIDS
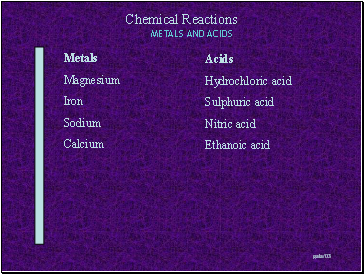
Metals
Magnesium
Iron
Sodium
Calcium
Slide 3
METALS AND ACIDS
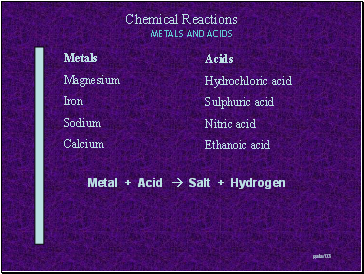
Metals
Magnesium
Iron
Sodium
Calcium
Acids
Hydrochloric acid
Sulphuric acid
Nitric acid
Ethanoic acid
Slide 4
METALS AND ACIDS
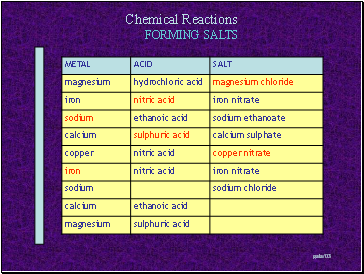
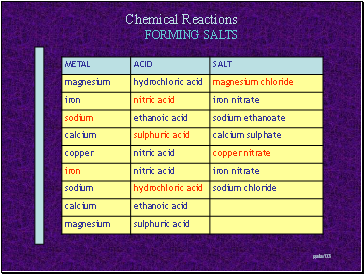
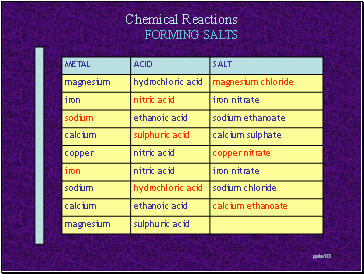
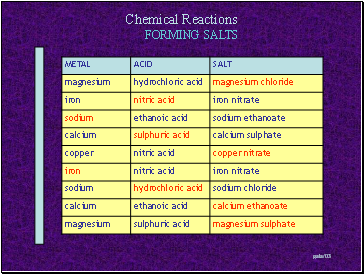
Metal + Acid Salt + Hydrogen
Metals
Magnesium
Iron
Sodium
Calcium
Acids
Hydrochloric acid
Sulphuric acid
Nitric acid
Ethanoic acid
Slide 5
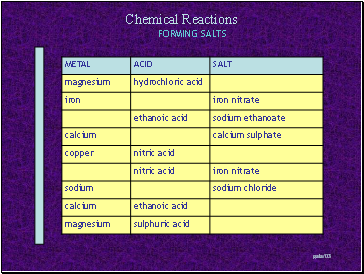
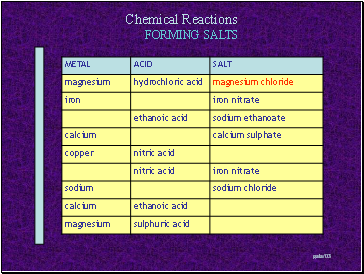
Forming salts
Slide 6
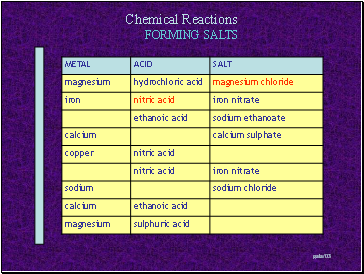
FORMING SALTS
Slide 7
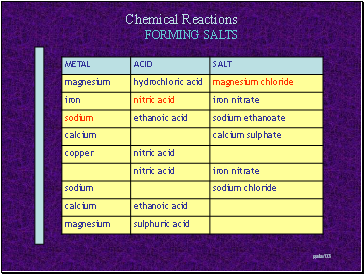
FORMING SALTS
Slide 8
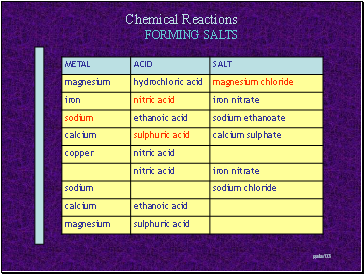
FORMING SALTS
Slide 9
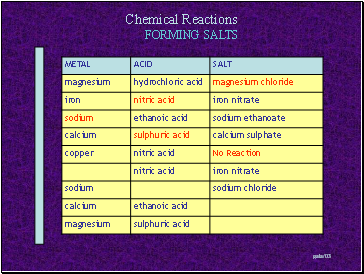
FORMING SALTS
Slide 10
FORMING SALTS
Slide 11
FORMING SALTS
Slide 12
FORMING SALTS
Slide 13
FORMING SALTS
Slide 14
FORMING SALTS
Slide 15
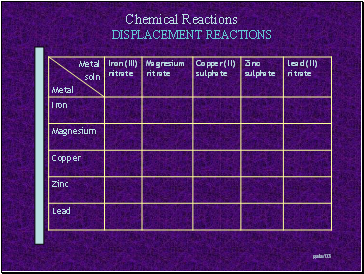
Displacement reactions
Slide 16
Displacement reactions
Instructions
Care! You should wear GOGGLES and LAB-COATS at all times during this practical
Obtain a dropping tile
Add a few drops of each of the solutions to individual ‘holes’ in the dropping tile. Make sure you know which solution is which
Choose a metal
Add one piece of that metal to each of the solutions
Observe – you will need to watch carefully for any evidence of a reaction taking place
If a reaction takes place, put a tick into the relevant box. If no reaction takes place, put a cross.
If a reaction takes place, try and describe what is happening
Wash all the reactions into a large beaker – do NOT put any solids into the sink!
Contents
Last added presentations
- Mechanics Lecture
- Heat-Energy on the Move
- Newton's laws of motion
- The Effects of Radiation on Living Things
- Radioactivity and Nuclear Reactions
- Health Physics
- Radiation