Properties of Acids BasesPage
2
2
H2SO4 + 2NaOH Na2SO4 + 2H2O
2HNO3 + Mg(OH)2 Mg(NO3)2 + 2H2O
Slide 13
Properties of Bases
Bases are proton (hydrogen ion, H+) acceptors
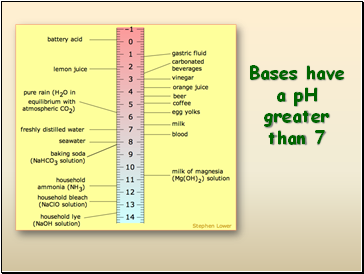
Bases have a pH greater than 7
Bases taste bitter
Bases effect indicators
Red litmus turns blue
Phenolphthalein turns purple
Solutions of bases feel slippery
Bases neutralize acids
Slide 14
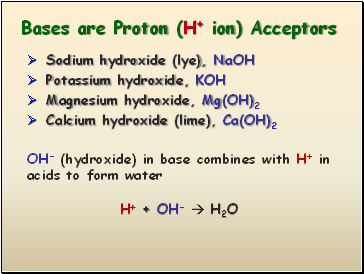
Bases are Proton (H+ ion) Acceptors
Sodium hydroxide (lye), NaOH
Potassium hydroxide, KOH
Magnesium hydroxide, Mg(OH)2
Calcium hydroxide (lime), Ca(OH)2
OH- (hydroxide) in base combines with H+ in acids to form water
H+ + OH- H2O
Slide 15
Bases have a pH greater than 7
Slide 16
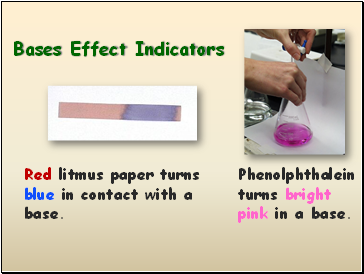
Bases Effect Indicators
Red litmus paper turns blue in contact with a base.
Phenolphthalein turns bright pink in a base.
Slide 17
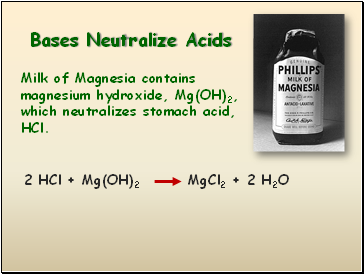
Bases Neutralize Acids
Milk of Magnesia contains magnesium hydroxide, Mg(OH)2, which neutralizes stomach acid, HCl.
2 HCl + Mg(OH)2
MgCl2 + 2 H2O
1 2
Contents
- Properties of Acids
- Acids are Proton (H+ ion) Donors
- Acids Taste Sour
- Organic Acids
- Acids Effect Indicators
- Acids React with Active Metals
- Acids React with Carbonates
- Acids Neutralize Bases
- Properties of Bases
- Bases are Proton (H+ ion) Acceptors
- Bases Effect Indicators
- Bases Neutralize Acids
Last added presentations
- Health Physics
- The Effects of Radiation on Living Things
- Newton’s laws of motion
- Gravitation
- Heat-Energy on the Move
- Practical Applications of Solar Energy
- Waves & Sound