StoichiometryPage
2
2
Three steps…Get Your Correct Answer
Use mole ratio
Get the answer in moles and then
Convert to Mass. [Simple Arithetic]
Hello!
If you are given a mass in the problem, you will need to convert this to moles first. Ok?
Slide 12
Let’s go!
2 KClO3 2 KCl + 3 O2
2 : 3
1.50 : X
X = 2.25mol
Convert to mass
2.25 mol x 32.0 g/mol = 72.0 grams
Cool!
Slide 13
Try This:
We want to produce 2.75 mol of KCl. How many grams of KClO3 would be required?
Soln
KClO3 : KCl
2 : 2
X : 2.75
X = 2.75mol
In mass: 2.75mol X 122.55 g/mol
= 337 grams zooo zimple!
Slide 14
Mass-Mass Problems
There are four steps involved in solving these problems:
Make sure you are working with a properly balanced equation.
Convert grams of the substance given in the problem to moles.
Construct two ratios - one from the problem and one from the equation and set them equal. Solve for "x," which is usually found in the ratio from the problem.
Convert moles of the substance just solved for into grams.
Slide 15
Mass-Volume Problems
Just follow mass-mass problem to the penultimate level
Slide 16
Like this:
There are four steps involved in solving these problems:
Make sure you are working with a properly balanced equation.
Convert grams of the substance given in the problem to moles.
Construct two ratios - one from the problem and one from the equation and set them equal. Solve for "x," which is usually found in the ratio from the problem.
Convert moles of the substance just solved for into Volume.
Slide 17
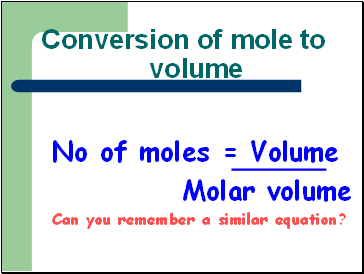
Conversion of mole to volume
No of moles = Volume
Molar volume
Can you remember a similar equation?
Slide 18
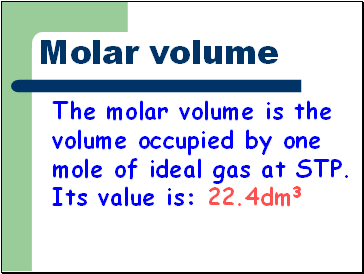
Molar volume
The molar volume is the volume occupied by one mole of ideal gas at STP. Its value is: 22.4dm3
Slide 19
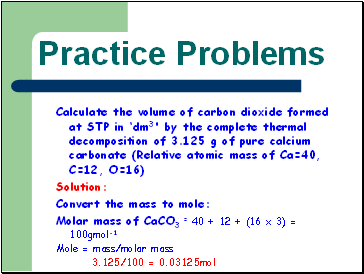
Practice Problems
Calculate the volume of carbon dioxide formed at STP in ‘dm3' by the complete thermal decomposition of 3.125 g of pure calcium carbonate (Relative atomic mass of Ca=40, C=12, O=16)
Solution:
Convert the mass to mole:
Molar mass of CaCO3 = 40 + 12 + (16 x 3) = 100gmol-1
Mole = mass/molar mass
Contents
- What is stoichiometry?
- Steps Involved in Solving Mass-Mass Stoichiometry Problems
- Mole Ratios
- Practice Problems
- Mole-Mole Problems
- Mole-Mass Problems
- Mass-Mass Problems
- Mass-Volume Problems
- Conversion of mole to volume
- Molar volume
- Practice Problems
Last added presentations
- Newton’s Laws of Motion
- Space Radiation
- Sound
- Heat-Energy on the Move
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Waves & Sound
- Direct heat utilization of geothermal energy