Bonds and EnergyPage
1
1
Slide 1
Understanding Chemical Reactions
Lesson: Bonds & Energy
Slide 2
Basic Revision
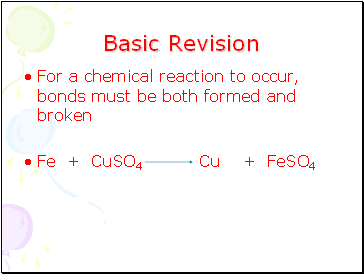
For a chemical reaction to occur, bonds must be both formed and broken
Fe + CuSO4 Cu + FeSO4
Slide 3
Basic Revision
Chemical reactions always involve energy changes.
Making and breaking bonds involves energy changes
Slide 4
Energy and chemical reactions
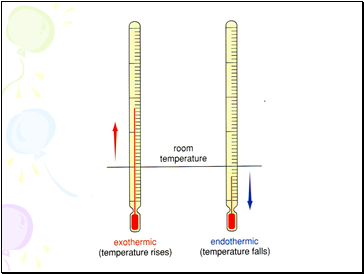
Exothermic reactions
These reactions give out heat energy.
Combustion is an exothermic reaction.
Slide 5
ENERGY AND CHEMICAL REACTIONS
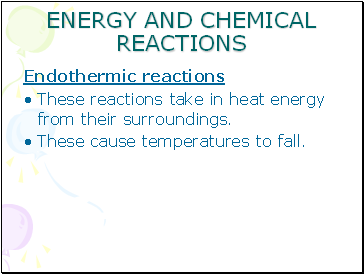
Endothermic reactions
These reactions take in heat energy from their surroundings.
These cause temperatures to fall.
Slide 6
Slide 7
ENERGY AND CHEMICAL REACTIONS
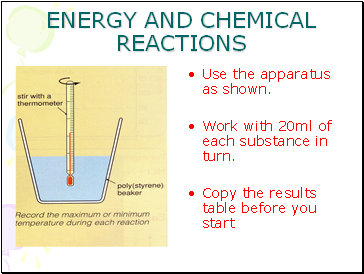
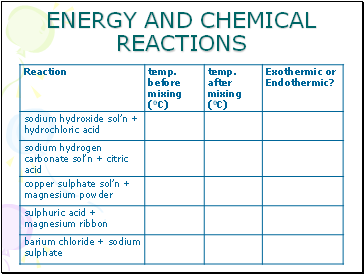
Use the apparatus as shown.
Work with 20ml of each substance in turn.
Copy the results table before you start
Slide 8
Risk assessment
You will be using acids so wear safety goggles.
Wipe up spills immediately
Replace stoppers
Do not put solids down the sink – use the bowl at the front!
Slide 9
ENERGY AND CHEMICAL REACTIONS
Slide 10
Energy level diagrams
We can show the energy transfers in reactions on an energy level diagram.
These show us the energy stored in the reactants compared to the energy stored in the products.
Slide 11
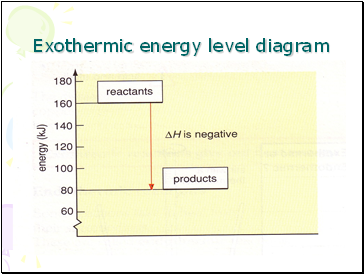
Exothermic energy level diagram
Slide 12
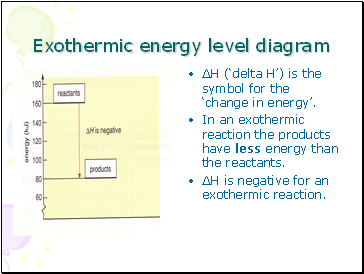
Exothermic energy level diagram
∆H (‘delta H’) is the symbol for the ‘change in energy’.
In an exothermic reaction the products have less energy than the reactants.
∆H is negative for an exothermic reaction.
Slide 13
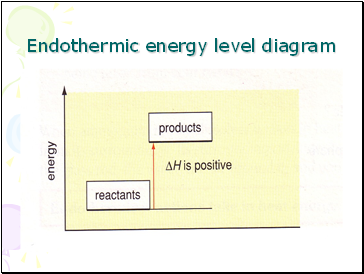
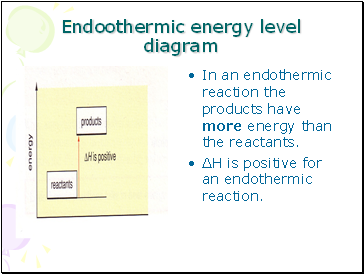
Endothermic energy level diagram
Slide 14
Endoothermic energy level diagram
In an endothermic reaction the products have more energy than the reactants.
1 2
Contents
- Basic Revision
- Energy and chemical reactions
- Risk assessment
- Energy level diagrams
- Exothermic energy level diagram
- Endothermic energy level diagram
- Making and breaking bonds
Last added presentations
- Buoyancy
- Upcoming Classes
- Mechanics Lecture
- Static and Kinetic Friction
- The Effects of Radiation on Living Things
- Solar Energy
- Sensory and Motor Mechanisms