BuffersPage
1
1
Slide 1
Buffers and Acid/Base Titration
Slide 2
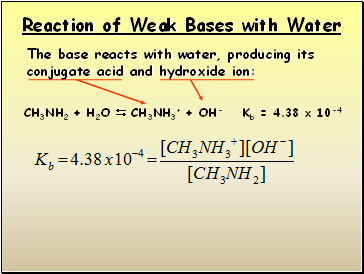
Reaction of Weak Bases with Water
The base reacts with water, producing its conjugate acid and hydroxide ion:
CH3NH2 + H2O CH3NH3+ + OH- Kb = 4.38 x 10-4
Slide 3
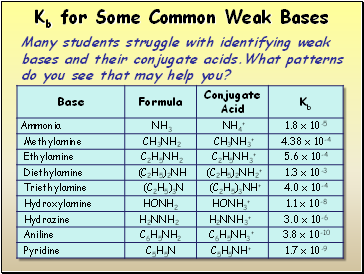
Kb for Some Common Weak Bases
Many students struggle with identifying weak bases and their conjugate acids.What patterns do you see that may help you?
Slide 4
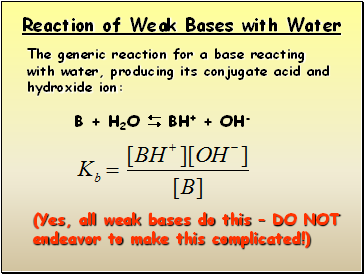
Reaction of Weak Bases with Water
The generic reaction for a base reacting with water, producing its conjugate acid and hydroxide ion:
B + H2O BH+ + OH-
(Yes, all weak bases do this – DO NOT
endeavor to make this complicated!)
Slide 5
Buffered Solutions
A solution that resists a change in pH when either hydroxide ions or protons are added.
Buffered solutions contain either:
A weak acid and its salt
A weak base and its salt
Slide 6
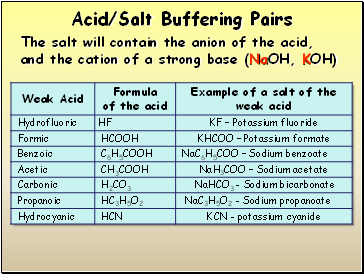
Acid/Salt Buffering Pairs
The salt will contain the anion of the acid, and the cation of a strong base (NaOH, KOH)
Slide 7
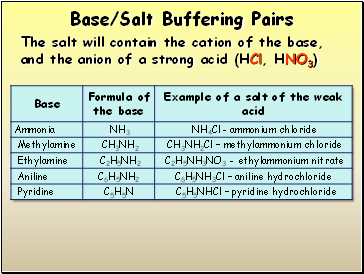
Base/Salt Buffering Pairs
The salt will contain the cation of the base, and the anion of a strong acid (HCl, HNO3)
Slide 8
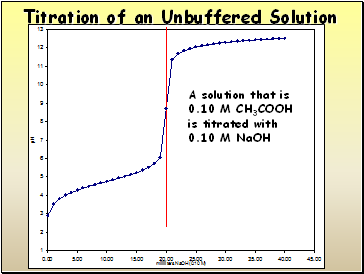
Titration of an Unbuffered Solution
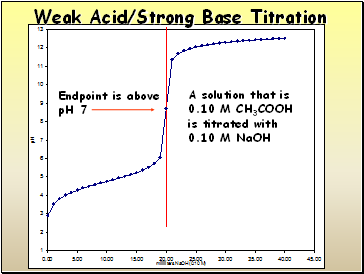
A solution that is
0.10 M CH3COOH
is titrated with
0.10 M NaOH
Slide 9
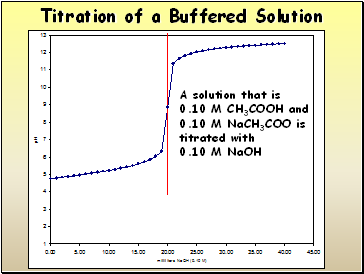
Titration of a Buffered Solution
A solution that is
0.10 M CH3COOH and
0.10 M NaCH3COO is titrated with
0.10 M NaOH
Slide 10
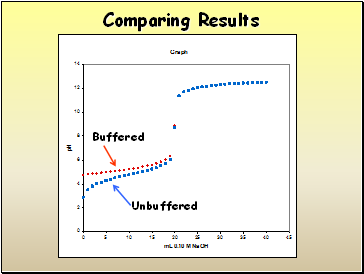
Comparing Results
Buffered
Unbuffered
Slide 11
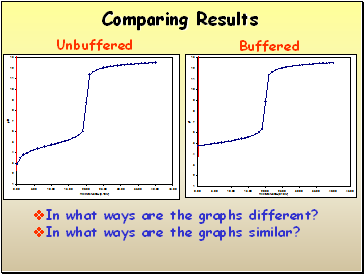
Comparing Results
Unbuffered
Buffered
In what ways are the graphs different?
In what ways are the graphs similar?
Slide 12
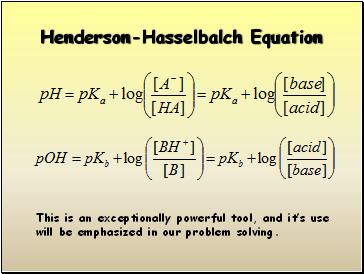
Henderson-Hasselbalch Equation
This is an exceptionally powerful tool, and it’s use will be emphasized in our problem solving.
Slide 13
Weak Acid/Strong Base Titration
1 2
Contents
- Reaction of Weak Bases with Water
- Kb for Some Common Weak Bases
- Reaction of Weak Bases with Water
- Buffered Solutions
- Acid/Salt Buffering Pairs
- Base/Salt Buffering Pairs
- Titration of an Unbuffered Solution
- Comparing Results
- Henderson-Hasselbalch Equation
- Weak Acid/Strong Base Titration
- Strong Acid/Strong Base Titration
- Selection of Indicators
- Indicator Transitions
Last added presentations
- Simulation at NASA for the Space Radiation Effort
- Practical Applications of Solar Energy
- The Effects of Radiation on Living Things
- Direct heat utilization of geothermal energy
- Space Radiation
- Newton's Laws
- Geophysical Concepts, Applications and Limitations