Green ChemistryPage
1
1
Slide 1
Green Chemistry
Slide 2
Green chemistry
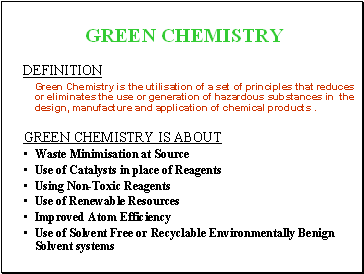
DEFINITION
Green Chemistry is the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products .
GREEN CHEMISTRY IS ABOUT
Waste Minimisation at Source
Use of Catalysts in place of Reagents
Using Non-Toxic Reagents
Use of Renewable Resources
Improved Atom Efficiency
Use of Solvent Free or Recyclable Environmentally Benign Solvent systems
Slide 3
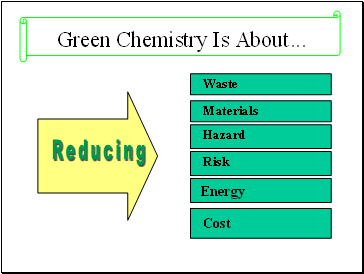
Green Chemistry Is About .
Cost
Waste
Materials
Hazard
Risk
Energy
Reducing
Slide 4
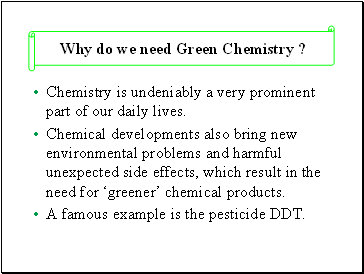
Why do we need Green Chemistry ?
Chemistry is undeniably a very prominent part of our daily lives.
Chemical developments also bring new environmental problems and harmful unexpected side effects, which result in the need for ‘greener’ chemical products.
A famous example is the pesticide DDT.
Slide 5
Green chemistry looks at pollution prevention on the molecular scale and is an extremely important area of Chemistry due to the importance of Chemistry in our world today and the implications it can show on our environment.
The Green Chemistry program supports the invention of more environmentally friendly chemical processes which reduce or even eliminate the generation of hazardous substances.
This program works very closely with the twelve principles of Green Chemistry.
Slide 6
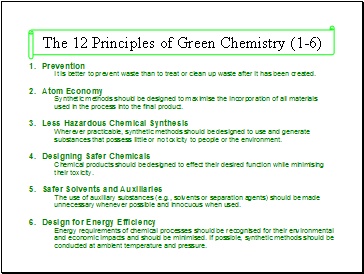
The 12 Principles of Green Chemistry (1-6)
Slide 7
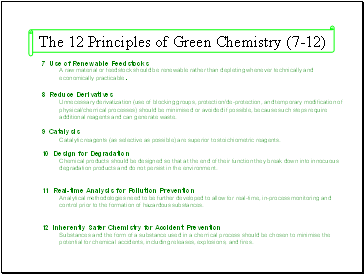
7 Use of Renewable Feedstocks
A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable.
8 Reduce Derivatives
Unnecessary derivatization (use of blocking groups, protection/de-protection, and temporary modification of physical/chemical processes) should be minimised or avoided if possible, because such steps require additional reagents and can generate waste.
9 Catalysis
Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
10 Design for Degradation
Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment.
11 Real-time Analysis for Pollution Prevention
Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances.
Contents
- Green chemistry
- Why do we need Green Chemistry ?
- The 12 Principles of Green Chemistry (1-6)
- Resource Depletion
- Polyhydroxyalkanoates (PHA’s)
- The major uses of GREEN CHEMISTRY
- Energy
- Global Change
- Resource Depletion
- Food Supply
- Toxics in the Environment
- Conclusion
Last added presentations
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Mechanics Lecture
- Newton's laws of motion
- Upcoming Classes
- History of Modern Astronomy
- Geophysical Concepts, Applications and Limitations
- Thermal Energy