Equilibrium and Le Chatelier's PrinciplePage
1
1
Slide 1
Equilibrium and Le Chatelierís Principle
Slide 2
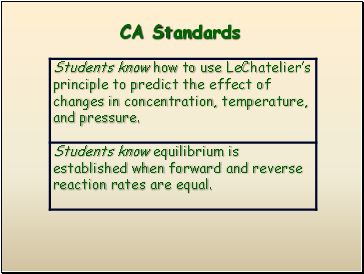
CA Standards
Slide 3
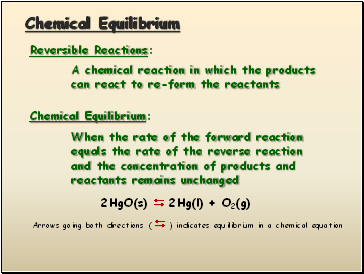
Chemical Equilibrium
Reversible Reactions:
A chemical reaction in which the products
can react to re-form the reactants
Chemical Equilibrium:
When the rate of the forward reaction
equals the rate of the reverse reaction
and the concentration of products and
reactants remains unchanged
2HgO(s) 2Hg(l) + O2(g)
Arrows going both directions ( ) indicates equilibrium in a chemical equation
Slide 4
LeChatelierís Principle
When a system at
equilibrium is placed under
stress, the system will
undergo a change in such
a way as to relieve that
stress.
Henry Le Chatelier
Slide 5
Le Chatelier Translated:
When you take something away from a system at equilibrium, the system shifts in such a way as to replace what youíve taken away.
When you add something to a system at equilibrium, the system shifts in such a way as to use up what youíve added.
Slide 6
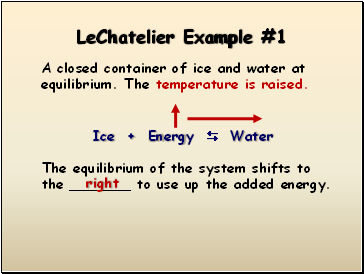
LeChatelier Example
#1
A closed container of ice and water at equilibrium. The temperature is raised.
Ice + Energy Water
The equilibrium of the system shifts to the _ to use up the added energy.
right
Slide 7
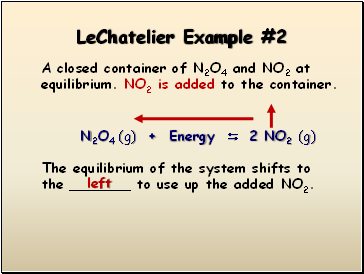
LeChatelier Example #2
A closed container of N2O4 and NO2 at equilibrium. NO2 is added to the container.
N2O4 (g) + Energy 2 NO2 (g)
The equilibrium of the system shifts to the _ to use up the added NO2.
left
Slide 8
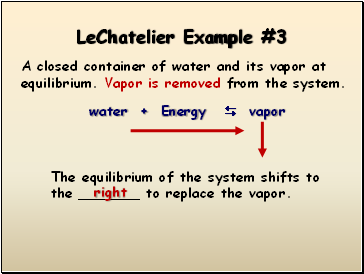
LeChatelier Example #3
A closed container of water and its vapor at equilibrium. Vapor is removed from the system.
water + Energy vapor
The equilibrium of the system shifts to the _ to replace the vapor.
right
Slide 9
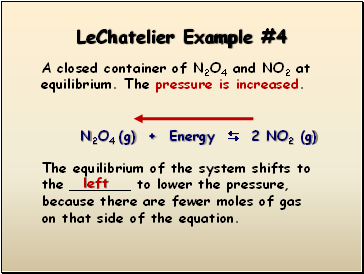
LeChatelier Example #4
A closed container of N2O4 and NO2 at equilibrium. The pressure is increased.
N2O4 (g) + Energy 2 NO2 (g)
The equilibrium of the system shifts to the _ to lower the pressure, because there are fewer moles of gas on that side of the equation.
left
Contents
Last added presentations
- History of Modern Astronomy
- Newton's laws of motion
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Gravitation
- Newtonís laws of motion
- Waves & Sound