Chemical reactionsPage
4
4
Cl2 was the limiting reactant.
Therefore, Al was present in excess. But how much?
First find how much Al was required.
Then find how much Al is in excess.
How much of which reactant will remain when reaction is complete?
Slide 36
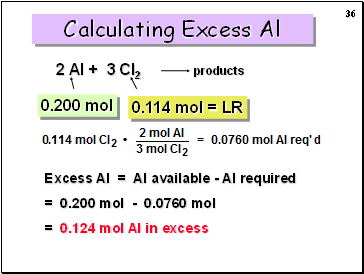
2 Al + 3 Cl2
products
0.200 mol
0.114 mol = LR
Calculating Excess Al
Excess Al = Al available - Al required
= 0.200 mol - 0.0760 mol
= 0.124 mol Al in excess
Slide 37
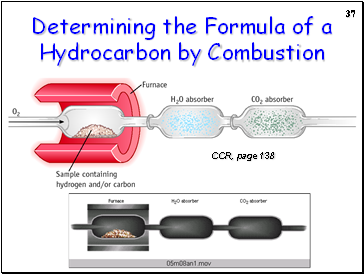
Determining the Formula of a Hydrocarbon by Combustion
CCR, page 138
Slide 38
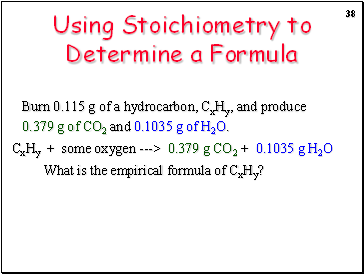
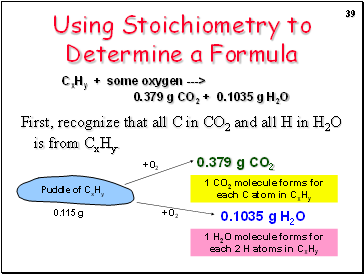
Using Stoichiometry to Determine a Formula
Burn 0.115 g of a hydrocarbon, CxHy, and produce 0.379 g of CO2 and 0.1035 g of H2O.
CxHy + some oxygen ---> 0.379 g CO2 + 0.1035 g H2O
What is the empirical formula of CxHy?
Slide 39
Using Stoichiometry to Determine a Formula
First, recognize that all C in CO2 and all H in H2O is from CxHy.
CxHy + some oxygen ---> 0.379 g CO2 + 0.1035 g H2O
0.379 g CO2
0.1035 g H2O
1 H2O molecule forms for each 2 H atoms in CxHy
1 CO2 molecule forms for each C atom in CxHy
Slide 40
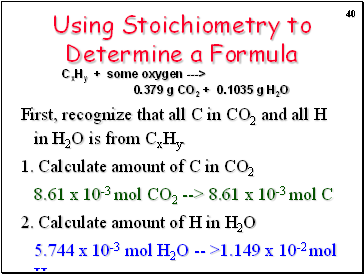
Using Stoichiometry to Determine a Formula
First, recognize that all C in CO2 and all H in H2O is from CxHy.
1. Calculate amount of C in CO2
8.61 x 10-3 mol CO2 --> 8.61 x 10-3 mol C
2. Calculate amount of H in H2O
5.744 x 10-3 mol H2O -- >1.149 x 10-2 mol H
CxHy + some oxygen ---> 0.379 g CO2 + 0.1035 g H2O
Slide 41
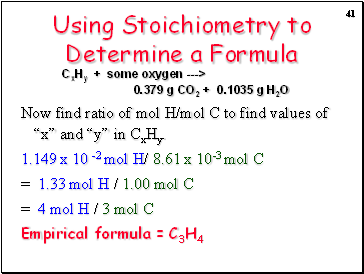
Using Stoichiometry to Determine a Formula
Now find ratio of mol H/mol C to find values of “x” and “y” in CxHy.
1.149 x 10 -2 mol H/ 8.61 x 10-3 mol C
= 1.33 mol H / 1.00 mol C
= 4 mol H / 3 mol C
Empirical formula = C3H4
CxHy + some oxygen ---> 0.379 g CO2 + 0.1035 g H2O
Contents
- Chemical Equations
- Balancing Equations
- Stoichiometry
- General plan for stoichiometry calculations
- Reactions Involving a Limiting reactant
- Limiting reactants
- Reaction to be Studied
- Using Stoichiometry to Determine a Formula
Last added presentations
- Madame Marie Curie
- Sound
- Radioactivity and Nuclear Reactions
- Health Physics
- Newton’s law of universal gravitation
- Radiation Safety and Operations
- Soil and Plant Nutrition