Jeopardy game- chemistryPage
2
2
Slide 14
Reactivity
Slide 15
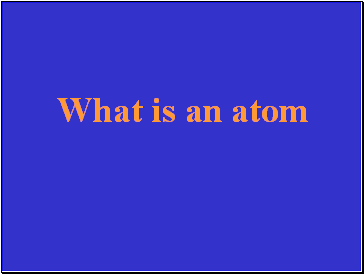
The smallest unit of a pure substance that still has the properties of that substance.
Slide 16
What is an atom
Slide 17
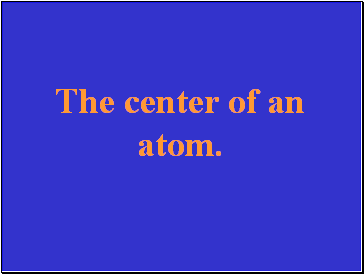
The center of an atom.
Slide 18
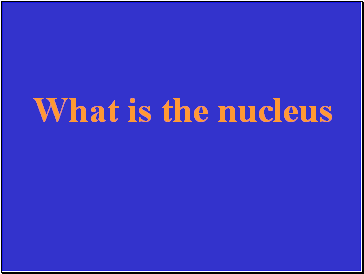
What is the nucleus
Slide 19
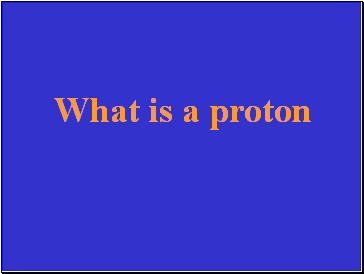
Every atomís nucleus contains at least one of these.
Slide 20
What is a proton
Slide 21
A negatively charged subatomic particle.
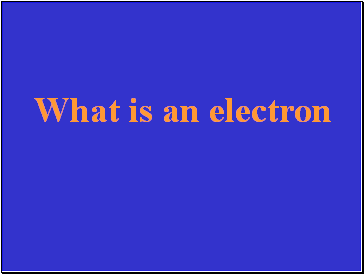
Slide 22
What is an electron
Slide 23
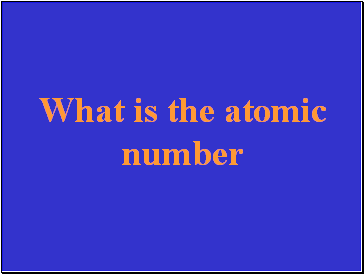
The number of protons in an atom.
Slide 24
What is the atomic number
Slide 25
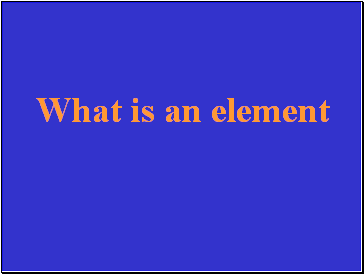
A pure substance that is made of only one kind of atom.
Slide 26
What is an element
Slide 27
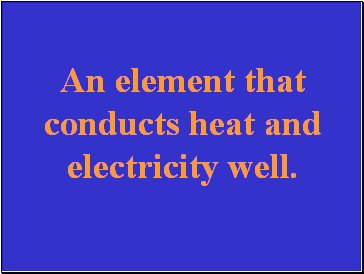
An element that conducts heat and electricity well.
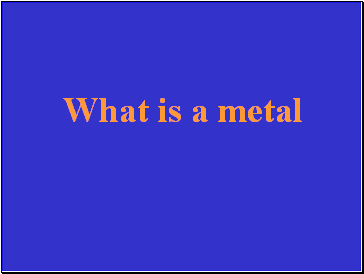
Slide 28
What is a metal
Slide 29
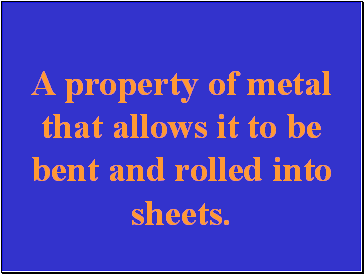
A property of metal that allows it to be bent and rolled into sheets.
Slide 30
What is malleability
Slide 31
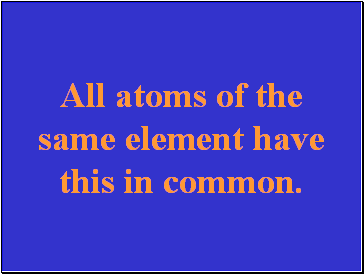
All atoms of the same element have this in common.
Slide 32
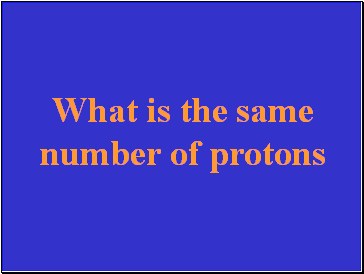
What is the same number of protons
Slide 33
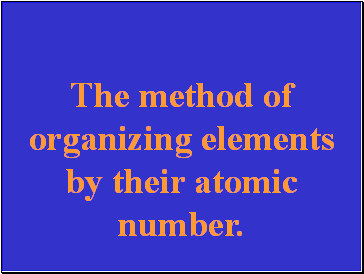
The method of organizing elements by their atomic number.
Slide 34
What is the periodic table
Slide 35
Contents
Last added presentations
- History of Modern Astronomy
- Newtonís laws of motion
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Newton's laws of motion
- Newtonís Law of Gravity
- Resource Acquisition and Transport in Vascular Plants
- The Effects of Radiation on Living Things