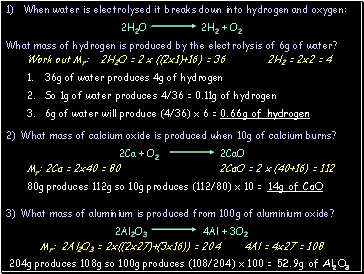Mass, atomic and empirical formulasPage
1
1
Slide 1
Relative mass formula, atomic mass, and empirical formula
Slide 2
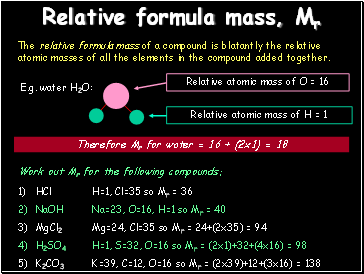
Relative formula mass, Mr
The relative formula mass of a compound is blatantly the relative atomic masses of all the elements in the compound added together.
E.g. water H2O:
Therefore Mr for water = 16 + (2x1) = 18
Work out Mr for the following compounds:
HCl
NaOH
MgCl2
H2SO4
K2CO3
H=1, Cl=35 so Mr = 36
Na=23, O=16, H=1 so Mr = 40
Mg=24, Cl=35 so Mr = 24+(2x35) = 94
H=1, S=32, O=16 so Mr = (2x1)+32+(4x16) = 98
K=39, C=12, O=16 so Mr = (2x39)+12+(3x16) = 138
Slide 3
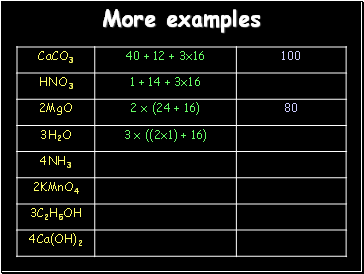
More examples
Slide 4
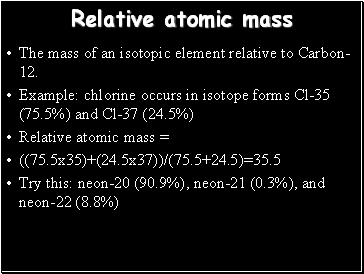
Relative atomic mass
The mass of an isotopic element relative to Carbon-12.
Example: chlorine occurs in isotope forms Cl-35 (75.5%) and Cl-37 (24.5%)
Relative atomic mass =
((75.5x35)+(24.5x37))/(75.5+24.5)=35.5
Try this: neon-20 (90.9%), neon-21 (0.3%), and neon-22 (8.8%)
Slide 5
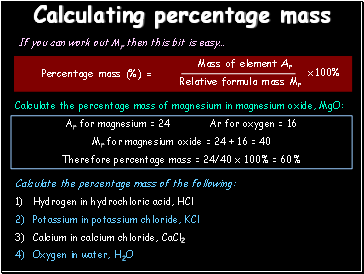
Calculating percentage mass
If you can work out Mr then this bit is easy…
Calculate the percentage mass of magnesium in magnesium oxide, MgO:
Ar for magnesium = 24 Ar for oxygen = 16
Mr for magnesium oxide = 24 + 16 = 40
Therefore percentage mass = 24/40 x 100% = 60%
Calculate the percentage mass of the following:
Hydrogen in hydrochloric acid, HCl
Potassium in potassium chloride, KCl
Calcium in calcium chloride, CaCl2
Oxygen in water, H2O
Slide 6
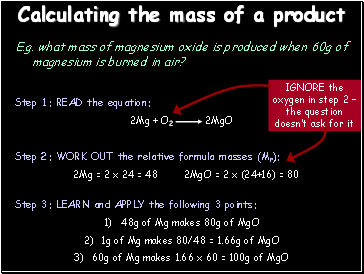
Calculating the mass of a product
E.g. what mass of magnesium oxide is produced when 60g of magnesium is burned in air?
Step 3: LEARN and APPLY the following 3 points:
48g of Mg makes 80g of MgO
1g of Mg makes 80/48 = 1.66g of MgO
60g of Mg makes 1.66 x 60 = 100g of MgO
Step 2: WORK OUT the relative formula masses (Mr):
2Mg = 2 x 24 = 48 2MgO = 2 x (24+16) = 80
Slide 7
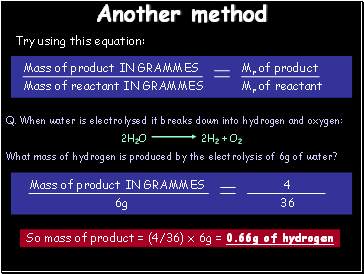
Work out Mr: 2H2O = 2 x ((2x1)+16) = 36 2H2 = 2x2 = 4
36g of water produces 4g of hydrogen
So 1g of water produces 4/36 = 0.11g of hydrogen
6g of water will produce (4/36) x 6 = 0.66g of hydrogen
Mr: 2Ca = 2x40 = 80 2CaO = 2 x (40+16) = 112
80g produces 112g so 10g produces (112/80) x 10 = 14g of CaO
Mr: 2Al2O3 = 2x((2x27)+(3x16)) = 204 4Al = 4x27 = 108
204g produces 108g so 100g produces (108/204) x 100 = 52.9g of Al2O3
Slide 8
Another method
1 2
Contents
- Relative formula mass, Mr
- More examples
- Relative atomic mass
- Calculating percentage mass
- Calculating the mass of a product
- Another method
- Calculating the volume of a product
- Example questions
- Empirical formulae
- Example questions
Last added presentations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Geophysical Concepts, Applications and Limitations
- Resource Acquisition and Transport in Vascular Plants
- Newton’s third law of motion
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Soil and Plant Nutrition
- Newton’s Laws of Motion