Light and electromagnetic spectrumPage
3
3
Slide 19
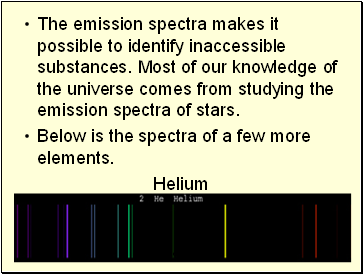
The emission spectra makes it possible to identify inaccessible substances. Most of our knowledge of the universe comes from studying the emission spectra of stars.
Below is the spectra of a few more elements.
Helium
Slide 20
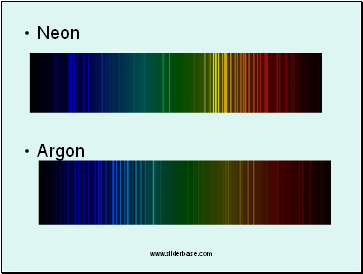
Neon
Argon
Slide 21
In a star, there are many elements present. The way we can tell which are there is to look at the spectrum of the star.
From spectral lines astronomers can determine not only the element, but the temperature and density of that element in the star
Emission lines can also tell us about the magnetic field of the star. The width of the line can tell us how fast the material is moving
Slide 22
If the lines shift back and forth, it means that the star may be orbiting another star - the spectrum will give the information to estimate the mass and size of the star system and the companion star.
Slide 23
Around a compact object (black hole, neutron star), the material is heated to the point it gives off X-rays, and the material falls onto the black hole or neutron star. By looking at the spectrum of X-rays being emitted by that object and its surrounding disk, we can learn about these objects.
Slide 24
Albert Einstein returned to the idea that light existed as particles. He proposed that light could be described as quanta of energy that behave as if they were particles. Light quanta are called
photons.
While it was difficult for scientists to believe (they can be stubborn) it did explain the photoelectric effect (previously a mystery)
Slide 25
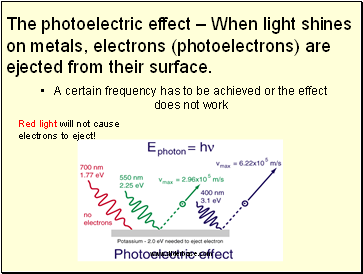
A certain frequency has to be achieved or the effect does not work
The photoelectric effect – When light shines on metals, electrons (photoelectrons) are ejected from their surface.
Red light will not cause electrons to eject!
Slide 26
The photoelectric effect has practical applications in photoelectrical cells used for solar powered cars, and solar powered calculators.
Contents
Last added presentations
- Upcoming Classes
- Motion
- Radioactivity and Nuclear Reactions
- Gravitation
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Waves & Sound
- Radiation