Light and electromagnetic spectrumPage
2
2
NOTE:
c is a constant value= 3.00 x 108 m/s
Slide 11
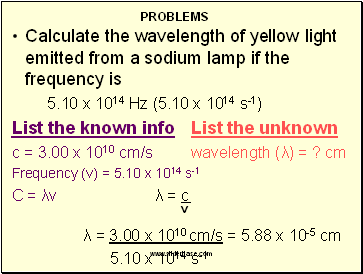
Calculate the wavelength of yellow light emitted from a sodium lamp if the frequency is
5.10 x 1014 Hz (5.10 x 1014 s-1)
List the known info List the unknown
c = 3.00 x 1010 cm/s wavelength (λ) = ? cm
Frequency (v) = 5.10 x 1014 s-1
C = λv λ = c
v
λ = 3.00 x 1010 cm/s = 5.88 x 10-5 cm
5.10 x 1014 s-1
PROBLEMS
Slide 12
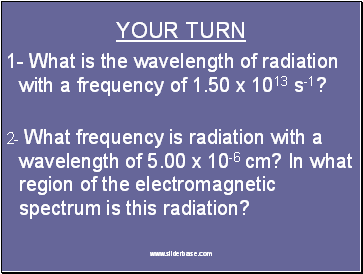
YOUR TURN
1- What is the wavelength of radiation with a frequency of 1.50 x 1013 s-1?
2- What frequency is radiation with a wavelength of 5.00 x 10-6 cm? In what region of the electromagnetic spectrum is this radiation?
Slide 13
The colors we see in objects are the colors that are reflected, all other colors are absorbed. A red t-shirt appears red because red is reflected to our eyes and the other colors are absorbed.
When all colors are being reflected we see white light (white isnít really a color)
Slide 14
When all wavelengths of light are being absorbed we see black (black also, isnít really a color)
A false-color image is made when the satellite records data about brightness of the light waves reflecting off the Earth's surface.
Slide 15
These brightnesses are represented by numerical values - and these values can then be color-coded. It is just like painting by number.
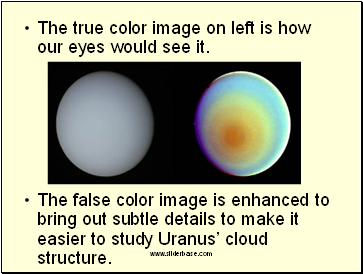
The next slide shows a true color vs. false color image of the planet Uranus. Satellite images can be gathered in true color (what our eyes would see) and false color (to make it look better)
Slide 16
The true color image on left is how our eyes would see it.
The false color image is enhanced to bring out subtle details to make it easier to study Uranusí cloud structure.
Slide 17
Atoms and Light
The movement of electrons inside of atoms produces light and other electromagnetic radiation.
Sunlight produces every color in the rainbow butÖ
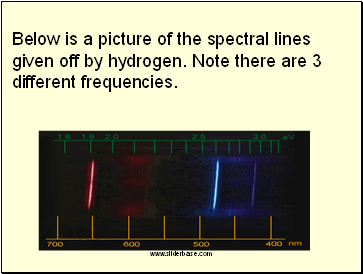
Each element gives off only certain frequencies of light, called spectral lines. In effect each element has its own signature of spectral lines allowing us to identify which element we have or what stars are made of.
Slide 18
Below is a picture of the spectral lines given off by hydrogen. Note there are 3 different frequencies.
Contents
Last added presentations
- Ch 9 Nuclear Radiation
- Friction
- Sound
- Solar Energy
- Mechanics Lecture
- Soil and Plant Nutrition
- Madame Marie Curie