Radioactivity and Nuclear ReactionsPage
5
5
Slide 40
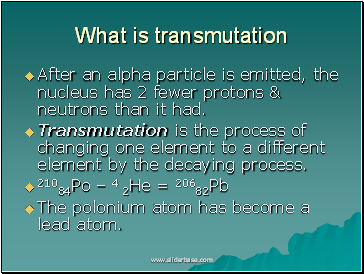
What is transmutation
After an alpha particle is emitted, the nucleus has 2 fewer protons & neutrons than it had.
Transmutation is the process of changing one element to a different element by the decaying process.
21084Po – 4 2He = 20682Pb
The polonium atom has become a lead atom.
Slide 41
Beta Particles
A second type of radioactive decay, beta radiation, a neutron decays into a proton by emitting an electron (0-1e). Beta decay is caused by the weak force.
Slide 42
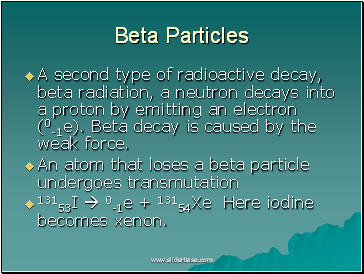
Beta Particles
A second type of radioactive decay, beta radiation, a neutron decays into a proton by emitting an electron (0-1e). Beta decay is caused by the weak force.
An atom that loses a beta particle undergoes transmutation
13153I 0-1e + 13154Xe Here iodine becomes xenon.
Slide 43
How can beta particles harm you?
Beta Particles are faster than alpha because they’re smaller & lighter so they penetrate deeper into material they hit.
Pass through paper
Aluminum foil will stop a beta particle
Can damage human cells if released inside the body
Slide 44
Gamma Rays
Gamma radiation is emitted as electromagnetic waves.
Gamma rays are EM waves with the highest frequencies & the shortest wavelength in the EM spectrum.
The symbol for a gamma ray is the Greek letter γ gamma.
Slide 45
Gamma Rays
Have no mass & no charge.
Travel at the speed of light.
Emitted by nucleus when alpha or beta particle is created.
Takes thick blocks of concrete or lead to stop gamma rays.
Cause less damage to cells inside the body than alpha or beta particles.
Slide 46
Radioactive Half-Life
The measure of the time it takes for half of the radioactive nuclei in a sample to decay is called a half-life.
The remaining nucleus is called the daughter nucleus.
Various isotopes decay at different rates.
Slide 47
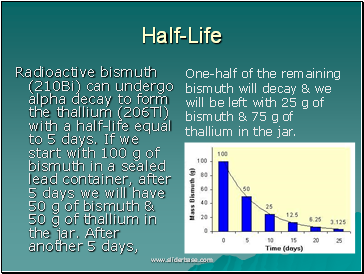
Half-Life
Radioactive bismuth (210Bi) can undergo alpha decay to form the thallium (206Tl) with a half-life equal to 5 days. If we start with 100 g of bismuth in a sealed lead container, after 5 days we will have 50 g of bismuth & 50 g of thallium in the jar. After another 5 days,
One-half of the remaining bismuth will decay & we will be left with 25 g of bismuth & 75 g of thallium in the jar.
Contents
- Radioactivity
- The Nucleus
- Is the nucleus the largest part of an atom?
- Modeling an Atom
- The Strong Force
- Radioactivity
- What are isotopes?
- What makes nuclei unstable?
- Nuclear Decay
- Nuclear Radiation
- Alpha Particles
- How can alpha particles harm you?
- What is transmutation
- Beta Particles
- Gamma Rays
- Radioactive Half-Life
- Half-Life
- Radioactive Dating
- Detecting Radioactivity
- Radiation Detectors
- What is a bubble chamber?
- Bubble Chamber
- Measuring Radiation
- Background Radiation
- Nuclear Reactions
- Nuclear Fission
- What is a chain reaction?
- Nuclear Fusion
- Fusion on the Sun
Last added presentations
- Simulation at NASA for the Space Radiation Effort
- Health Physics
- Radioactivity and Nuclear Reactions
- Space Radiation
- Mechanics Lecture
- Direct heat utilization of geothermal energy
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal