Solution StoichiometryPage
1
1
Slide 1
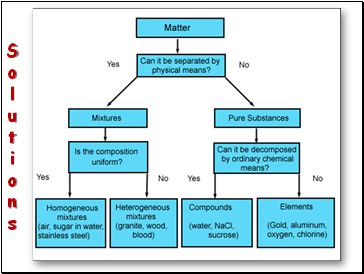
Solutions
Slide 2
Solute
A solute is the dissolved substance in a solution.
A solvent is the dissolving medium in a solution.
Solvent
Salt in salt water
Sugar in soda drinks
Carbon dioxide in soda drinks
Water in salt water
Water in soda
Slide 3
“Like Dissolves Like”
Polar and ionic solutes dissolve best in polar solvents
Nonpolar solutes dissolve best in nonpolar solvents
Slide 4
Solubility Trends
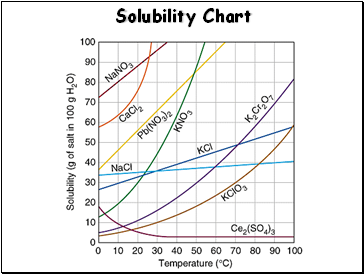
The solubility of MOST solids increases with temperature.
The rate at which solids dissolve increases with increasing surface area of the solid.
The solubility of gases decreases with increases in temperature.
The solubility of gases increases with the pressure above the solution.
Slide 5
Therefore…
Solids tend to dissolve best when:
Heated
Stirred
Ground into small particles
Gases tend to dissolve best when:
The solution is cold
Pressure is high
Slide 6
Solubility Chart
Slide 7
Saturation of Solutions
A solution that contains the maximum amount of solute that may be dissolved under existing conditions is saturated.
A solution that contains less solute than a saturated solution under existing conditions is unsaturated.
A solution that contains more dissolved solute than a saturated solution under the same conditions is supersaturated.
Slide 8
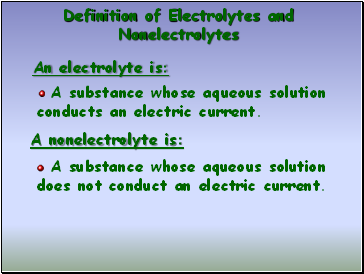
Definition of Electrolytes and Nonelectrolytes
An electrolyte is:
A substance whose aqueous solution conducts an electric current.
A nonelectrolyte is:
A substance whose aqueous solution does not conduct an electric current.
Slide 9
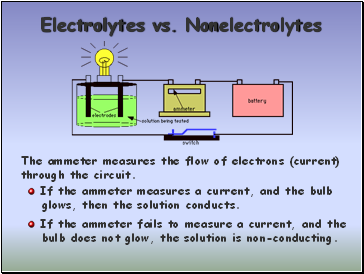
Electrolytes vs. Nonelectrolytes
The ammeter measures the flow of electrons (current)
through the circuit.
If the ammeter measures a current, and the bulb
glows, then the solution conducts.
If the ammeter fails to measure a current, and the
bulb does not glow, the solution is non-conducting.
Slide 10
Pure water
Tap water
Sugar solution
Sodium chloride solution
1 2
Contents
- Solutions
- Solubility Trends
- Therefore…
- Solubility Chart
- Saturation of Solutions
- Definition of Electrolytes and Nonelectrolytes
- Electrolytes vs. Nonelectrolytes
- Answers to Electrolytes
- Ionic Compounds “Dissociate”
- Some covalent compounds IONIZE in solution
Last added presentations
- Sound
- Sensory and Motor Mechanisms
- Newton’s Laws of Motion
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Buoyancy
- Solar Energy
- Health Physics