The Structure and Properties of PolymersPage
1
1
Slide 1
The Structure and Properties of Polymers
Also known as
Bonding +
Properties
Slide 2
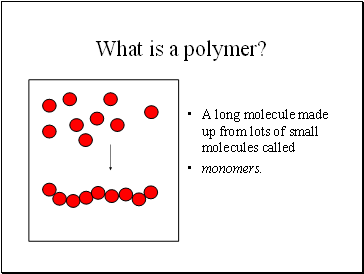
What is a polymer?
A long molecule made up from lots of small molecules called
monomers.
Slide 3
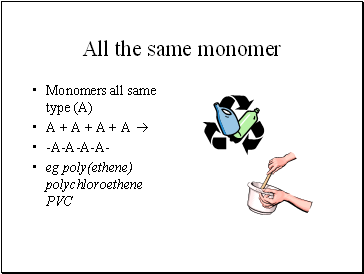
All the same monomer
Monomers all same type (A)
A + A + A + A
-A-A-A-A-
eg poly(ethene) polychloroethene PVC
Slide 4
Different monomers
Monomers of two different types A + B
A + B + A + B
-A-B-A-B-
eg polyamides
polyesters
Slide 5
Addition polymerisation
Monomers contain C=C bonds
Double bond opens to (link) bond to next monomer molecule
Chain forms when same basic unit is repeated over and over.
Modern polymers also developed based on alkynes R-C C - R’
Slide 6
Copolymerisation
when more than one monomer is used.
An irregular chain structure will result eg propene/ethene/propene/propene/ethene
Why might polymers designers want to design a polymer in this way?
(Hint) Intermolecular bonds!
Slide 7
Elastomers, plastics & fibres
Find a definition and suggest your own example of each of these.
Slide 8
What decides the properties of a polymer?
Stronger attractive forces between chains = stronger, less flexible polymer.
Chains able to slide past each other = flexible polymer .
In poly(ethene) attractive forces are weak instantaneous dipole - induced dipole, will it be flexible or not?
Nylon has strong hydrogen bonds, why does this make it a strong fibre?
Slide 9
Getting ideas straight
Look at page 110 -111 of Chemical Ideas.
Take turns in explaining to a partner how the following molecular structures affect the overall properties of polymers :-
chain length, different side groups, chain branching, stereoregularity, chain flexibility, cross linking.
Slide 10
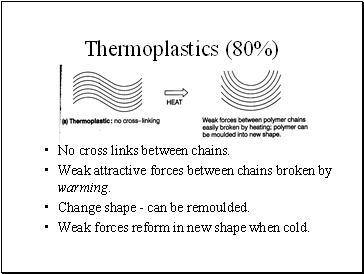
Thermoplastics (80%)
No cross links between chains.
Weak attractive forces between chains broken by warming.
Change shape - can be remoulded.
Weak forces reform in new shape when cold.
1 2
Contents
- What is a polymer?
- All the same monomer
- Different monomers
- Addition polymerisation
- Copolymerisation
- Elastomers, plastics & fibres
- What decides the properties of a polymer?
- Getting ideas straight
- Thermoplastics (80%)
- Thermosets
- Longer chains make stronger polymers.
- Crystalline polymers
- Cold-drawing
Last added presentations
- Newton’s third law of motion
- Ch 9 Nuclear Radiation
- Newton's Laws
- Soil and Plant Nutrition
- Friction
- Heat-Energy on the Move
- Simulation at NASA for the Space Radiation Effort