Acids, pH and EquilibriumPage
2
2
A Weak Acid Equilibrium Problem
What is the pH of a 0.50 M solution of acetic acid, HC2H3O2, Ka = 1.8 x 10-5 ?
Step #2: ICE it!
HC2H3O2 C2H3O2- + H+
0.50
0
0
- x
+x
+x
0.50 - x
x
x
Slide 14
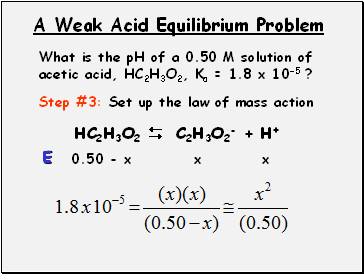
A Weak Acid Equilibrium Problem
What is the pH of a 0.50 M solution of acetic acid, HC2H3O2, Ka = 1.8 x 10-5 ?
Step #3: Set up the law of mass action
HC2H3O2 C2H3O2- + H+
0.50 - x
x
x
E
Slide 15
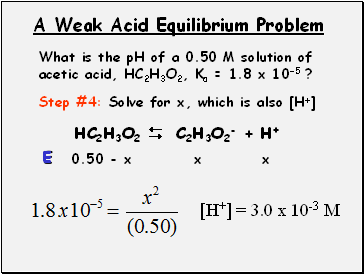
A Weak Acid Equilibrium Problem
What is the pH of a 0.50 M solution of acetic acid, HC2H3O2, Ka = 1.8 x 10-5 ?
Step #4: Solve for x, which is also [H+]
HC2H3O2 C2H3O2- + H+
0.50 - x
x
x
E
[H+] = 3.0 x 10-3 M
Slide 16
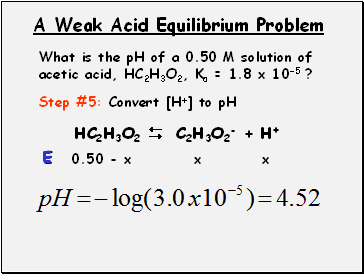
A Weak Acid Equilibrium Problem
What is the pH of a 0.50 M solution of acetic acid, HC2H3O2, Ka = 1.8 x 10-5 ?
Step #5: Convert [H+] to pH
HC2H3O2 C2H3O2- + H+
0.50 - x
x
x
E
Go to page:
1 2
1 2
Contents
- Acid Equilibrium and pH
- Acid/Base Definitions
- Acid Dissociation
- Dissociation of Strong Acids
- Dissociation Constants: Strong Acids
- Dissociation of Weak Acids
- Dissociation Constants: Weak Acids
- Self-Ionization of Water
- A Weak Acid Equilibrium Problem
Last added presentations
- Solar Thermal Energy
- Magnetic field uses sound waves to ignite sun's ring of fire
- Solar Energy
- Newton's laws of motion
- Motion
- Practical Applications of Solar Energy
- History of Modern Astronomy
© 2010-2026 powerpoint presentations