BuffersPage
2
2
A solution that is
0.10 M CH3COOH
is titrated with
0.10 M NaOH
Endpoint is above pH 7
Slide 14
Strong Acid/Strong Base Titration
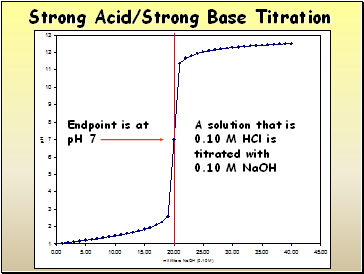
A solution that is
0.10 M HCl is titrated with
0.10 M NaOH
Endpoint is at
pH 7
Slide 15
Strong Acid/Strong Base Titration
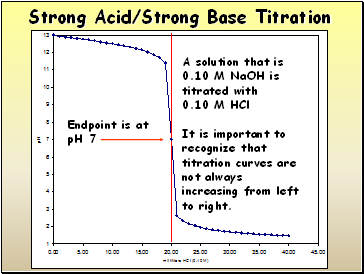
A solution that is
0.10 M NaOH is titrated with
0.10 M HCl
Endpoint is at
pH 7
It is important to recognize that titration curves are not always increasing from left to right.
Slide 16
Strong Acid/Weak Base Titration
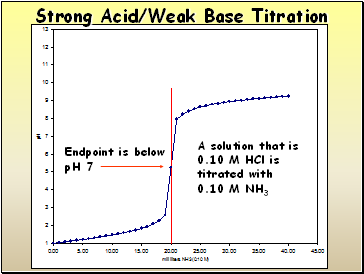
A solution that is
0.10 M HCl is titrated with
0.10 M NH3
Endpoint is below
pH 7
Slide 17
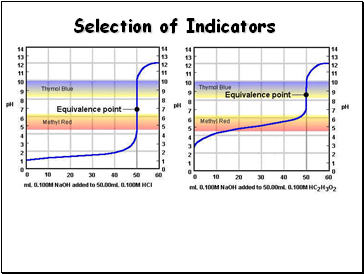
Selection of Indicators
Slide 18
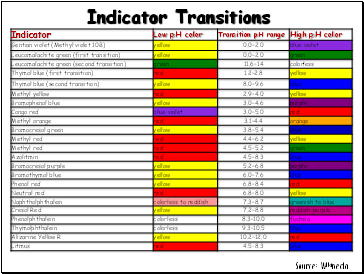
Indicator Transitions
Source: Wikipedia
Go to page:
1 2
1 2
Contents
- Reaction of Weak Bases with Water
- Kb for Some Common Weak Bases
- Reaction of Weak Bases with Water
- Buffered Solutions
- Acid/Salt Buffering Pairs
- Base/Salt Buffering Pairs
- Titration of an Unbuffered Solution
- Comparing Results
- Henderson-Hasselbalch Equation
- Weak Acid/Strong Base Titration
- Strong Acid/Strong Base Titration
- Selection of Indicators
- Indicator Transitions
Last added presentations
- Newton’s law of universal gravitation
- Madame Marie Curie
- Radiation
- Mechanics Lecture
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Solar Thermal Energy
- Thermal Energy
© 2010-2025 powerpoint presentations