Electronic configurationPage
1
1
Slide 1
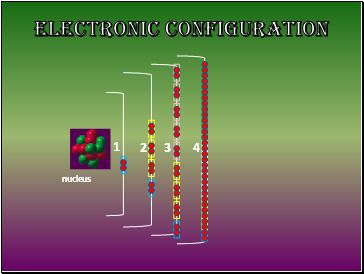
Electronic Configuration
nucleus
Slide 2
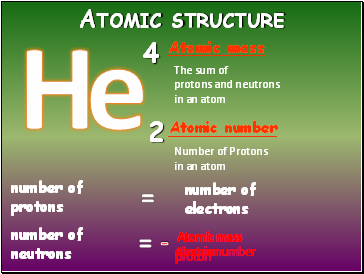
Atomic structure
4
Atomic mass
2
Atomic number
number of
electrons
number of
protons
=
The sum of
protons and neutrons
in an atom
Number of Protons
in an atom
Atomic mass
Atomic number
-
Atomic mass
proton
-
Atomic mass
electron
-
Slide 3
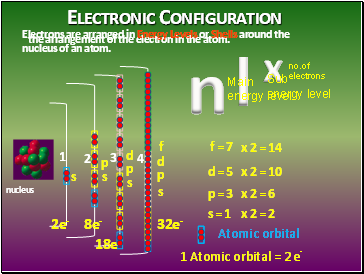
Electronic Configuration
the arrangement of the electron in the atom.
Electrons are arranged in Energy Levels or Shells around the nucleus of an atom.
nucleus
n
l
x
s
d
f
s
p
s
p
d
p
s
Atomic orbital
f = 7
d = 5
p = 3
s = 1
1 Atomic orbital = 2 e-
x 2 = 2
x 2 = 6
x 2 = 10
x 2 = 14
2e-
8e-
32e-
Main energy level
Sub
energy level
no.of
electrons
18e-
Slide 4
Learning Check
Given the
Element
Complete the
missing data.
p+
e-
n0
Number of
Location in the
atom
1)
2)
3)
4)
5)
6)
Slide 5
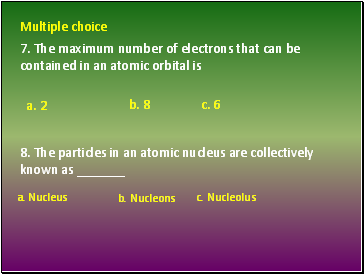
7. The maximum number of electrons that can be contained in an atomic orbital is
8. The particles in an atomic nucleus are collectively known as _
Multiple choice
a. 2
a. Nucleus c. Nucleolus
b. Nucleons
b. 8 c. 6
Slide 6
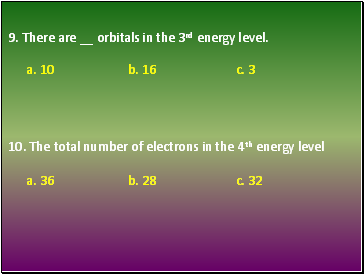
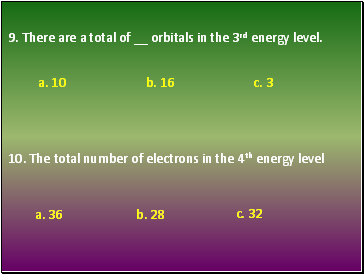
9. There are orbitals in the 3rd energy level.
10. The total number of electrons in the 4th energy level
a. 10 b. 16 c. 3
a. 36 b. 28 c. 32
Slide 7
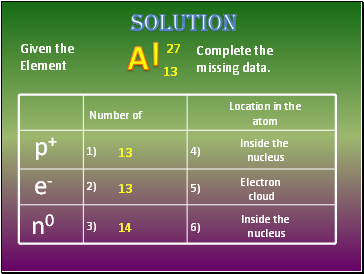
Given the
Element
Complete the
missing data.
p+
e-
n0
Number of
Location in the
atom
1)
2)
3)
4)
5)
6)
solution
13
13
14
Inside the
nucleus
Inside the
nucleus
Electron
cloud
Slide 8
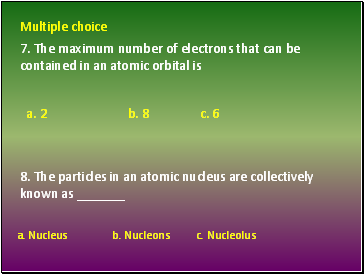
Multiple choice
a. Nucleus c. Nucleolus
7. The maximum number of electrons that can be contained in an atomic orbital is
8. The particles in an atomic nucleus are collectively known as _
b. 8 c. 6
b. Nucleons
a. 2
Slide 9
9. There are a total of orbitals in the 3rd energy level.
10. The total number of electrons in the 4th energy level
1 2
Contents
Last added presentations
- Sound
- Mechanics Lecture
- Magnetic field uses sound waves to ignite sun's ring of fire
- Friction
- Soil and Plant Nutrition
- Solar Thermal Energy
- Solar Energy