Acids and BasesPage
2
2
Slide 10
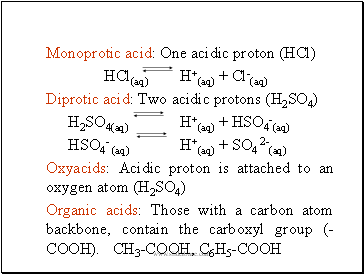
Monoprotic acid: One acidic proton (HCl)
HCl(aq) H+(aq) + Cl-(aq)
Diprotic acid: Two acidic protons (H2SO4)
H2SO4(aq) H+(aq) + HSO4-(aq)
HSO4- (aq) H+(aq) + SO4 2-(aq)
Oxyacids: Acidic proton is attached to an oxygen atom (H2SO4)
Organic acids: Those with a carbon atom backbone, contain the carboxyl group (-COOH). CH3-COOH, C6H5-COOH
Slide 11
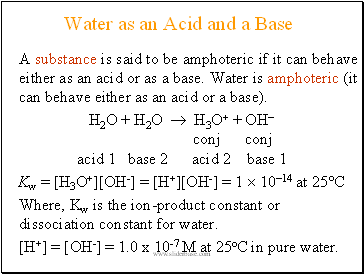
Water as an Acid and a Base
A substance is said to be amphoteric if it can behave either as an acid or as a base. Water is amphoteric (it can behave either as an acid or a base).
H2O + H2O H3O+ + OH
conj conj
acid 1 base 2 acid 2 base 1
Kw = [H3O+][OH-] = [H+][OH-] = 1 1014 at 25°C
Where, Kw is the ion-product constant or dissociation constant for water.
[H+] = [OH-] = 1.0 x 10-7 M at 25oC in pure water.
Slide 12
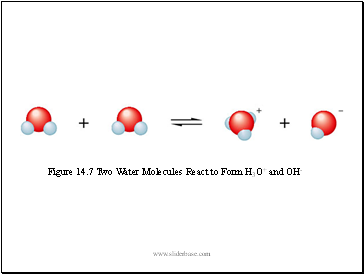
Figure 14.7 Two Water Molecules React to Form H3O+ and OH-
Slide 13
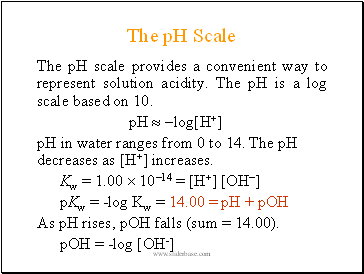
The pH Scale
The pH scale provides a convenient way to represent solution acidity. The pH is a log scale based on 10.
pH log[H+]
pH in water ranges from 0 to 14. The pH decreases as [H+] increases.
Kw = 1.00 1014 = [H+] [OH]
pKw = -log Kw = 14.00 = pH + pOH
As pH rises, pOH falls (sum = 14.00).
pOH = -log [OH-]
Slide 14
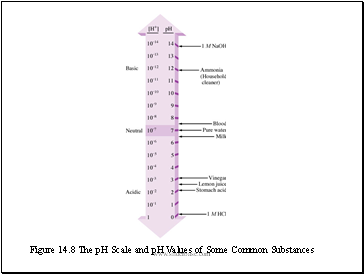
Figure 14.8 The pH Scale and pH Values of Some Common Substances
Slide 15
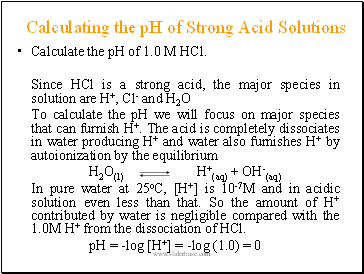
Calculating the pH of Strong Acid Solutions
Calculate the pH of 1.0 M HCl.
Since HCl is a strong acid, the major species in solution are H+, Cl- and H2O
To calculate the pH we will focus on major species that can furnish H+. The acid is completely dissociates in water producing H+ and water also furnishes H+ by autoionization by the equilibrium
H2O(l) H+(aq) + OH-(aq)
In pure water at 25oC, [H+] is 10-7M and in acidic solution even less than that. So the amount of H+ contributed by water is negligible compared with the 1.0M H+ from the dissociation of HCl.
pH = -log [H+] = -log (1.0) = 0
Slide 16
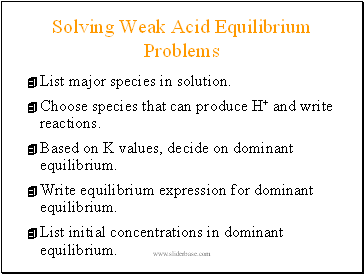
Solving Weak Acid Equilibrium Problems
List major species in solution.
Choose species that can produce H+ and write reactions.
Based on K values, decide on dominant equilibrium.
Write equilibrium expression for dominant equilibrium.
List initial concentrations in dominant equilibrium.
Contents
- Acids and Bases
- Conjugate Acid/Base Pairs
- Acid Dissociation Constant (Ka)
- Acid Strength
- Water as an Acid and a Base
- The pH Scale
- Calculating the pH of Strong Acid Solutions
- Solving Weak Acid Equilibrium Problems
- Percent Dissociation (Ionization)
- Bases
- Polyprotic Acids
- Acid-Base Properties of Salts
- Structure and Acid-Base Properties
- Oxides
- Lewis Acids and Bases
Last added presentations
- Sound
- Thermal Energy
- Practical Applications of Solar Energy
- Newton's Laws
- Simulation at NASA for the Space Radiation Effort
- Solar Thermal Energy
- Mechanics Lecture
