Atomic StructurePage
1
1
Slide 1
Chapter 41
Atomic Structure
Slide 2
Goals for Chapter 41
To see how to write the Schrödinger equation for a three-dimensional problem
To learn how to find the wave functions and energies for a three-dimensional box
To examine the full quantum-mechanical description of the hydrogen atom
To study how an external magnetic field affects the orbital motion of an atom’s electrons
To learn about the intrinsic angular momentum (spin) of the electron
To understand how the exclusion principle affects the structure of many-electron atoms
To study how the x-ray spectra of atoms indicate the structure of these atoms
Slide 3
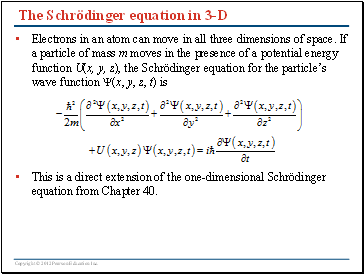
The Schrödinger equation in 3-D
Electrons in an atom can move in all three dimensions of space. If a particle of mass m moves in the presence of a potential energy function U(x, y, z), the Schrödinger equation for the particle’s wave function (x, y, z, t) is
This is a direct extension of the one-dimensional Schrödinger equation from Chapter 40.
Slide 4
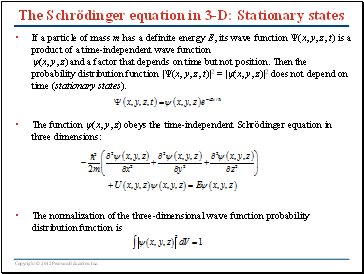
The Schrödinger equation in 3-D: Stationary states
If a particle of mass m has a definite energy E, its wave function (x, y, z, t) is a product of a time-independent wave function (x, y, z) and a factor that depends on time but not position. Then the probability distribution function |(x, y, z, t)|2 = |(x, y, z)|2 does not depend on time (stationary states).
The function (x, y, z) obeys the time-independent Schrödinger equation in three dimensions:
The normalization of the three-dimensional wave function probability distribution function is
Slide 5
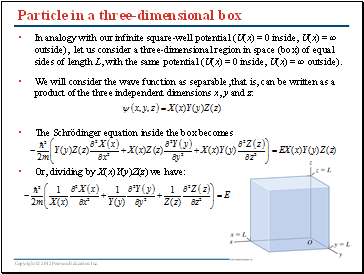
Particle in a three-dimensional box
In analogy with our infinite square-well potential (U(x) = 0 inside, U(x) = ∞ outside), let us consider a three-dimensional region in space (box) of equal sides of length L, with the same potential (U(x) = 0 inside, U(x) = ∞ outside).
We will consider the wave function as separable, that is, can be written as a product of the three independent dimensions x, y and z:
The Schrödinger equation inside the box becomes
Or, dividing by X(x)Y(y)Z(z) we have:
Slide 6
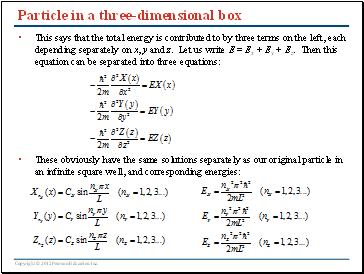
Particle in a three-dimensional box
This says that the total energy is contributed to by three terms on the left, each depending separately on x, y and z. Let us write E = Ex + Ey + Ez. Then this equation can be separated into three equations:
Contents
- The Schrödinger equation in 3-D
- Particle in a three-dimensional box
- Energy Degeneracy
- The hydrogen atom: Quantum numbers
- Description of solution
- Quantization of Angular Momentum
- The hydrogen atom: Degeneracy
- The hydrogen atom: Quantum states
- The hydrogen atom: Probability distributions I
- Magnetic moments and the Zeeman effect
- The Zeeman effect and selection rules
- The anomalous Zeeman effect and electron spin
- Electron spin and the Stern-Gerlach experiment
- Quantum states and the Pauli exclusion principle
- A multielectron atom
- Ground-state electron configurations
- Screening in multielectron atoms
- X-ray spectroscopy
Last added presentations
- Sound
- Friction
- Direct heat utilization of geothermal energy
- Waves & Sound
- Newton’s laws of motion
- Simulation at NASA for the Space Radiation Effort
- Magnetic field uses sound waves to ignite sun's ring of fire