Atomic StructurePage
2
2
These obviously have the same solutions separately as our original particle in an infinite square well, and corresponding energies:
Slide 7
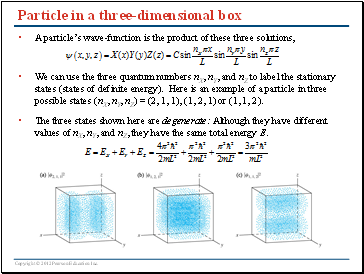
Particle in a three-dimensional box
A particle’s wave-function is the product of these three solutions,
We can use the three quantum numbers nX, nY, and nZ to label the stationary states (states of definite energy). Here is an example of a particle in three possible states (nX, nY, nZ) = (2, 1, 1), (1, 2, 1) or (1, 1, 2).
The three states shown here are degenerate: Although they have different values of nX, nY, and nZ, they have the same total energy E.
Slide 8
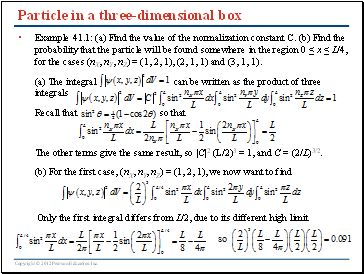
Particle in a three-dimensional box
Example 41.1: (a) Find the value of the normalization constant C. (b) Find the probability that the particle will be found somewhere in the region 0 ≤ x ≤ L/4, for the cases (nX, nY, nZ) = (1, 2, 1), (2, 1, 1) and (3, 1, 1).
(a) The integral can be written as the product of three integrals
Recall that so that
The other terms give the same result, so |C|2 (L/2)3 = 1, and C = (2/L)3/2.
(b) For the first case, (nX, nY, nZ) = (1, 2, 1), we now want to find
Only the first integral differs from L/2, due to its different high limit
Slide 9
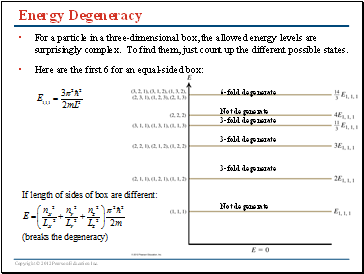
Energy Degeneracy
For a particle in a three-dimensional box, the allowed energy levels are surprisingly complex. To find them, just count up the different possible states.
Here are the first 6 for an equal-sided box:
6-fold degenerate
Not degenerate
3-fold degenerate
3-fold degenerate
3-fold degenerate
Not degenerate
Slide 10
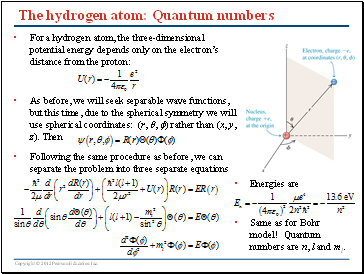
The hydrogen atom: Quantum numbers
Energies are
Same as for Bohr model! Quantum numbers are n, l and ml.
For a hydrogen atom, the three-dimensional potential energy depends only on the electron’s distance from the proton:
As before, we will seek separable wave functions, but this time, due to the spherical symmetry we will use spherical coordinates: (r, , ) rather than (x, y, z). Then
Following the same procedure as before, we can separate the problem into three separate equations
Slide 11
Description of solution
We will not go through the mathematics of the solution, but note that we can only accept solutions for which the wave function is normalizable (does not blow up). Some radial solutions blow up at r = 0 or r = ∞, and so must be discarded. As in the harmonic oscillator problem, the radial solutions R(r) turn out to be an exponential function e-ar, multiplied by a polynomial in r. The angular solutions Q(q) are polynomials containing powers of sinq and cosq. The azimuthal solutions F(f) have to be periodic (have the same value at F(f) and F(f+2p), etc. They turn out to depend on eimlf, where ml is an integer, either positive, negative or zero.
Contents
- The Schrödinger equation in 3-D
- Particle in a three-dimensional box
- Energy Degeneracy
- The hydrogen atom: Quantum numbers
- Description of solution
- Quantization of Angular Momentum
- The hydrogen atom: Degeneracy
- The hydrogen atom: Quantum states
- The hydrogen atom: Probability distributions I
- Magnetic moments and the Zeeman effect
- The Zeeman effect and selection rules
- The anomalous Zeeman effect and electron spin
- Electron spin and the Stern-Gerlach experiment
- Quantum states and the Pauli exclusion principle
- A multielectron atom
- Ground-state electron configurations
- Screening in multielectron atoms
- X-ray spectroscopy
Last added presentations
- Solar Thermal Energy
- History of Modern Astronomy
- Direct heat utilization of geothermal energy
- Upcoming Classes
- Madame Marie Curie
- Geophysical Concepts, Applications and Limitations
- Practical Applications of Solar Energy