Atoms and the Periodic tablePage
8
8
Slide 71
Learning Check
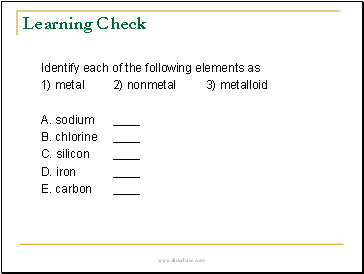
Identify each of the following elements as
1) metal 2) nonmetal 3) metalloid
A. sodium
B. chlorine
C. silicon
D. iron
E. carbon
Slide 72
Solution
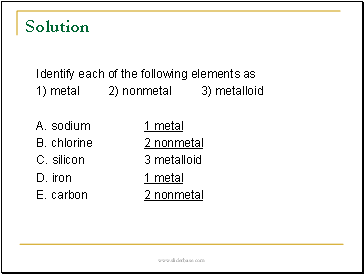
Identify each of the following elements as
1) metal 2) nonmetal 3) metalloid
A. sodium 1 metal
B. chlorine 2 nonmetal
C. silicon 3 metalloid
D. iron 1 metal
E. carbon 2 nonmetal
Slide 73
Learning Check
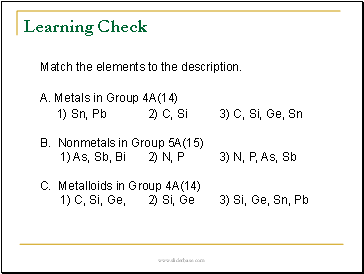
Match the elements to the description.
A. Metals in Group 4A(14)
1) Sn, Pb 2) C, Si 3) C, Si, Ge, Sn
B. Nonmetals in Group 5A(15)
1) As, Sb, Bi 2) N, P 3) N, P, As, Sb
C. Metalloids in Group 4A(14)
1) C, Si, Ge, 2) Si, Ge 3) Si, Ge, Sn, Pb
Slide 74
Solution
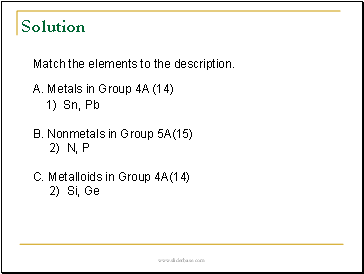
Match the elements to the description.
A. Metals in Group 4A (14)
1) Sn, Pb
B. Nonmetals in Group 5A(15)
2) N, P
C. Metalloids in Group 4A(14)
2) Si, Ge
Slide 75
Exploration of the Periodic Table/ Periodic Reactivity Trends
Run the following web animations/movies.
3.4b:
3.6:
Slide 76
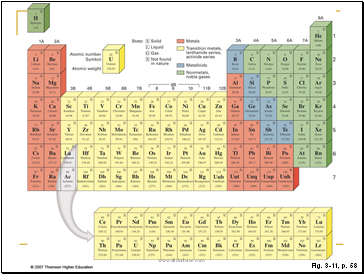
Figure 3.11: Modern periodic table of elements.
Slide 77
Slide 78
Three elements: sulfur (bottom) and diamond (which is carbon, top left), which are main-group nonmetals; and gold (top right), a transition metal.
Slide 79
Chapter Outline
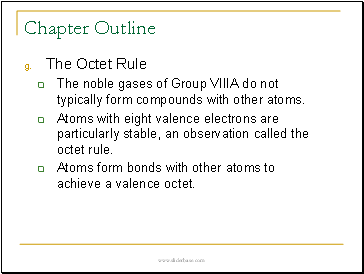
• The Octet Rule
The noble gases of Group VIIIA do not typically form compounds with other atoms.
Atoms with eight valence electrons are particularly stable, an observation called the octet rule.
Atoms form bonds with other atoms to achieve a valence octet.
Slide 80
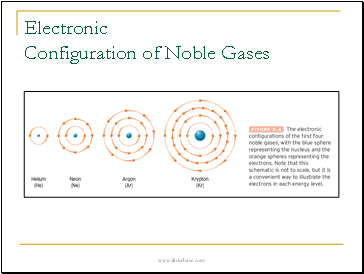
Electronic Configuration of Noble Gases
Slide 81
Chapter Outline
Lewis Dot Structures
The number of valence electrons is equal to the group number for most of the main group elements.
In Lewis dot structures, the chemical symbol represents the nucleus and the core electrons and dots represent the valence electrons.
Contents
- Atoms and the periodic table
- Conservation of Matter
- Atomic Line Spectra
- Atomic Subshell Energies
- Exploration of the Periodic Table/ Periodic Reactivity Trends
Last added presentations
- Motion
- Friction
- Heat-Energy on the Move
- Gravitation
- Radiation Safety and Operations
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Solar Energy