Atoms and the Periodic tablePage
6
6
Learning Check
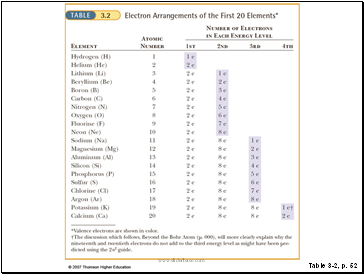
State the number of valence electrons for each.
A. 2, 8, 5
B. 2, 8, 8, 2
C. 2, 7
Slide 46
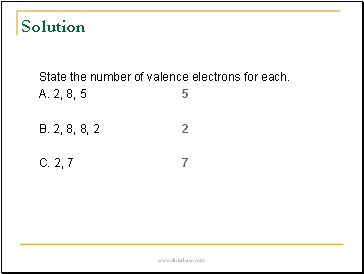
Solution
State the number of valence electrons for each.
A. 2, 8, 5 5
B. 2, 8, 8, 2 2
C. 2, 7 7
Slide 47
Energy levels are spaced differently, like ladder rungs
Slide 48
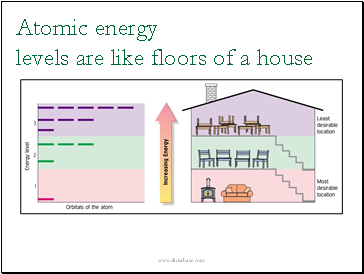
Atomic energy levels are like floors of a house
Slide 49
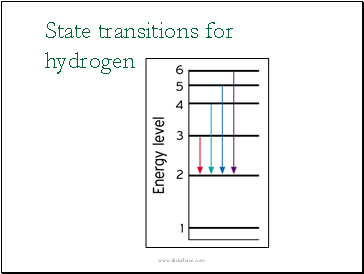
State transitions for hydrogen
Slide 50
Slide 51
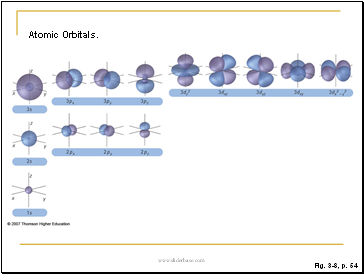
Figure 3.8: Atomic orbitals.
Boundary surface diagrams for electron densities of 1s, 2s, 2p, 3s, 3p, and 3d orbitals. For the p orbitals, the subscript letter on the orbital notation (x, y, z) indicates the cartesian axis along which the orbital lies.
Slide 52
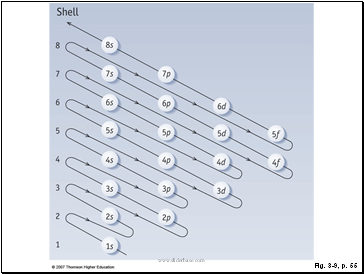
Figure 3.9: Subshell filling order.
Subshells in atoms are filled in order of increasing energy, as this diagram shows. The order of filling is 1s → 2s → 2p → 3s → 3p → 4s → 3d and so on.
Slide 53
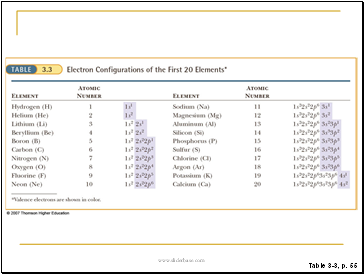
Atomic Subshell Energies
Run the following web animations/movies.
3.3:
Slide 54
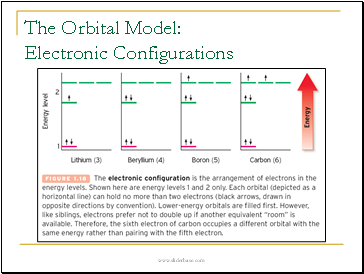
The Orbital Model: Electronic Configurations
Slide 55
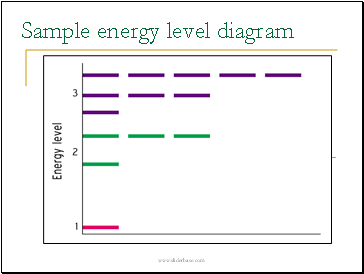
Sample energy level diagram
Slide 56
Slide 57
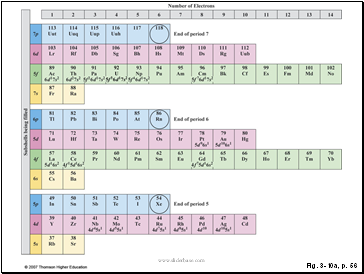
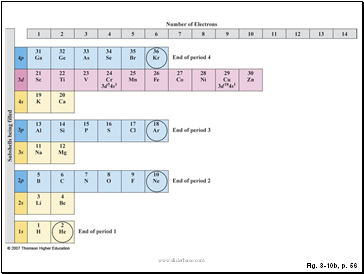
Figure 3.10: In this “building-up” version of the periodic table, the lightest elements are at the bottom. Electrons fill subshells from bottom to top in order of energy as the atomic number of the atom increases. The numbers across the top give the number of electrons in each subshell. The ground-state electron configurations of most elements are apparent from their positions in the table. Those that are known to differ from expectation are indicated explicitly.
Slide 58
Figure 3.10: In this “building-up” version of the periodic table, the lightest elements are at the bottom. Electrons fill subshells from bottom to top in order of energy as the atomic number of the atom increases. The numbers across the top give the number of electrons in each subshell. The ground-state electron configurations of most elements are apparent from their positions in the table. Those that are known to differ from expectation are indicated explicitly.
Contents
- Atoms and the periodic table
- Conservation of Matter
- Atomic Line Spectra
- Atomic Subshell Energies
- Exploration of the Periodic Table/ Periodic Reactivity Trends
Last added presentations
- Sensory and Motor Mechanisms
- Simulation at NASA for the Space Radiation Effort
- Sound
- Direct heat utilization of geothermal energy
- Radioactivity and Nuclear Reactions
- Static and Kinetic Friction
- Madame Marie Curie