Atoms and their structurePage
5
5
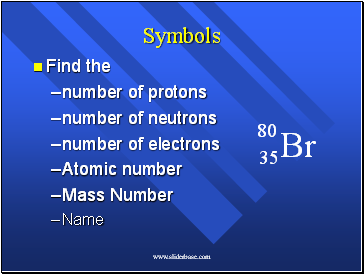
Symbols
Find the
number of protons
number of neutrons
number of electrons
Atomic number
Mass Number
Name
Na
24
11
Slide 49
Symbols
Find the
number of protons
number of neutrons
number of electrons
Atomic number
Mass Number
Name
Br
80
35
Slide 50
Symbols
if an element has an atomic number of 34 and a mass number of 78 what is the
number of protons
number of neutrons
number of electrons
Complete symbol
Name
Slide 51
Symbols
if an element has 91 protons and 140 neutrons what is the
Atomic number
Mass number
number of electrons
Complete symbol
Name
Slide 52
Symbols
if an element has 78 electrons and 117 neutrons what is the
Atomic number
Mass number
number of protons
Complete symbol
Name
Slide 53
Atomic Mass
How heavy is an atom of oxygen?
There are different kinds of oxygen atoms.
More concerned with average atomic mass.
Based on abundance of each element in nature.
Don’t use grams because the numbers would be too small.
Slide 54
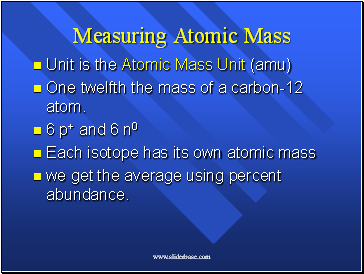
Measuring Atomic Mass
Unit is the Atomic Mass Unit (amu)
One twelfth the mass of a carbon-12 atom.
6 p+ and 6 n0
Each isotope has its own atomic mass
we get the average using percent abundance.
Slide 55
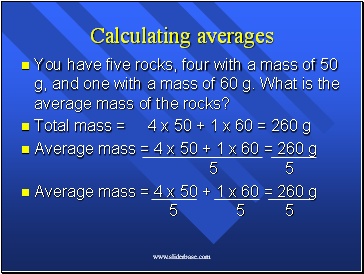
Calculating averages
You have five rocks, four with a mass of 50 g, and one with a mass of 60 g. What is the average mass of the rocks?
Total mass = 4 x 50 + 1 x 60 = 260 g
Average mass = 4 x 50 + 1 x 60 = 260 g 5 5
Average mass = 4 x 50 + 1 x 60 = 260 g 5 5 5
Slide 56
Calculating averages
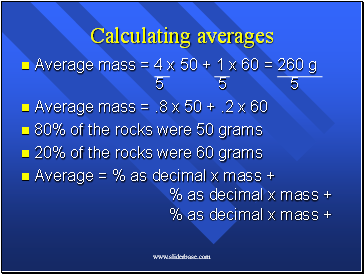
Average mass = 4 x 50 + 1 x 60 = 260 g 5 5 5
Average mass = .8 x 50 + .2 x 60
80% of the rocks were 50 grams
20% of the rocks were 60 grams
Average = % as decimal x mass + % as decimal x mass + % as decimal x mass +
Slide 57
Atomic Mass
Calculate the atomic mass of copper if copper has two isotopes. 69.1% has a mass of 62.93 amu and the rest has a mass of 64.93 amu.
Slide 58
Contents
- History of the atom
- Another Greek
- Who Was Right?
- Who’s Next?
- Dalton’s Atomic Theory
- Parts of Atoms
- Thomsom’s Model
- Rutherford’s Experiment
- He Expected
- Modern View
- Density and the Atom
- Structure of the Atom
- Size of an atom
- Counting the Pieces
- Isotopes
- Naming Isotopes
- Atomic Mass
- Calculating averages
Last added presentations
- Geophysical Concepts, Applications and Limitations
- Newton’s Law of Gravity
- Magnetic field uses sound waves to ignite sun's ring of fire
- Sensory and Motor Mechanisms
- Newton's laws of motion
- Direct heat utilization of geothermal energy
- Mechanics Lecture