Hybridization of OrbitalsPage
1
1
Slide 1
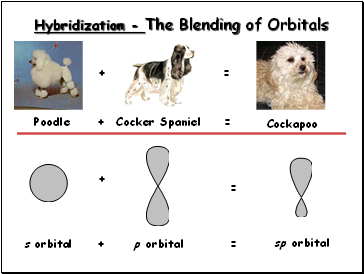
Hybridization - The Blending of Orbitals
Poodle
+
+
Cocker Spaniel
=
=
=
=
+
+
s orbital
p orbital
Cockapoo
sp orbital
Slide 2
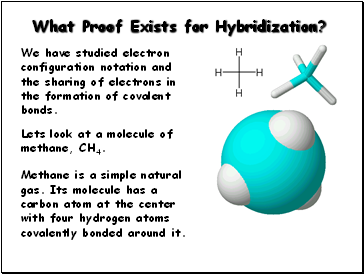
What Proof Exists for Hybridization?
We have studied electron configuration notation and
the sharing of electrons in the formation of covalent
bonds.
Methane is a simple natural gas. Its molecule has a
carbon atom at the center with four hydrogen atoms covalently bonded around it.
Lets look at a molecule of methane, CH4.
Slide 3
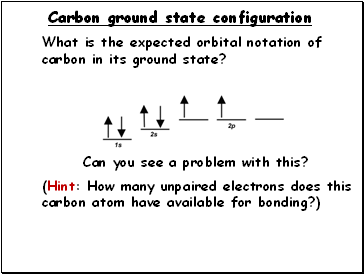
Carbon ground state configuration
What is the expected orbital notation of carbon in its ground state?
(Hint: How many unpaired electrons does this
carbon atom have available for bonding?)
Can you see a problem with this?
Slide 4
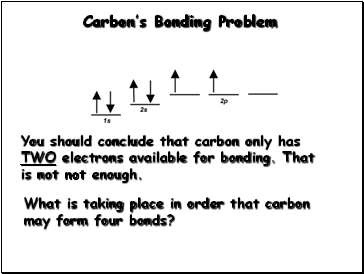
Carbon’s Bonding Problem
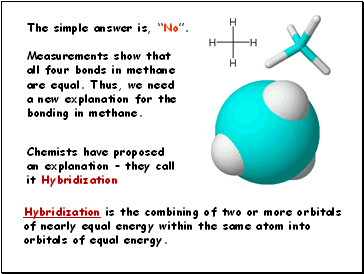
You should conclude that carbon only has TWO electrons available for bonding. That is not not enough.
What is taking place in order that carbon may form four bonds?
Slide 5
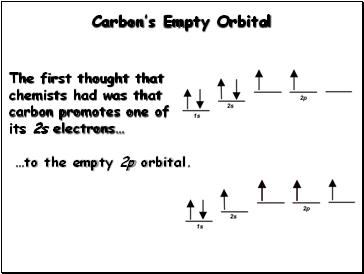
Carbon’s Empty Orbital
The first thought that chemists had was that carbon promotes one of its 2s electrons…
…to the empty 2p orbital.
Slide 6
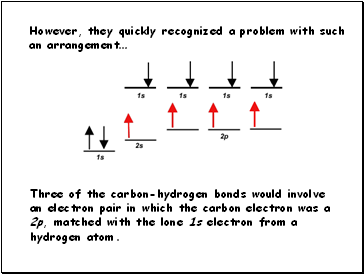
However, they quickly recognized a problem with such
an arrangement…
Three of the carbon-hydrogen bonds would involve
an electron pair in which the carbon electron was a 2p, matched with the lone 1s electron from a hydrogen atom.
Slide 7
This would mean that three of the bonds in a methane
molecule would be identical, because they would involve
electron pairs of equal energy.
But what about the fourth bond…?
Slide 8
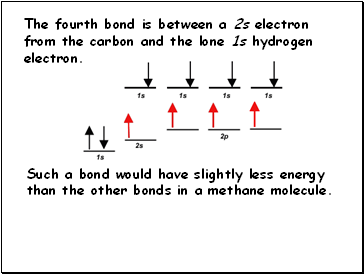
The fourth bond is between a 2s electron from the carbon and the lone 1s hydrogen electron.
Such a bond would have slightly less energy than the other bonds in a methane molecule.
Slide 9
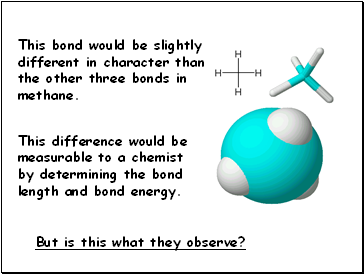
This bond would be slightly different in character than
the other three bonds in methane.
This difference would be measurable to a chemist
by determining the bond length and bond energy.
But is this what they observe?
Slide 10
Contents
- Hybridization - The Blending of Orbitals
- What Proof Exists for Hybridization?
- Carbon ground state configuration
- Carbon’s Bonding Problem
- Carbon’s Empty Orbital
- Hybrid Orbitals
- Exclusion Warning
- Hybridization Involving “d” Orbitals
- Hybridization and Molecular Geometry
- Sigma and Pi Bonds
- Sigma and Pi Bonds
- The De-Localized Electron Model
Last added presentations
- Heat-Energy on the Move
- Ch 9 Nuclear Radiation
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Gravitation
- Resource Acquisition and Transport in Vascular Plants
- Simulation at NASA for the Space Radiation Effort
- Mechanics Lecture