The Building Blocks of Matter AtomsPage
2
2
http://www.miamisci.org/af/sln/phantom/papercutting.html
Slide 7
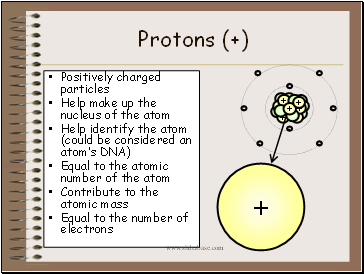
Protons (+)
Positively charged particles
Help make up the nucleus of the atom
Help identify the atom (could be considered an atom’s DNA)
Equal to the atomic number of the atom
Contribute to the atomic mass
Equal to the number of electrons
+
Slide 8
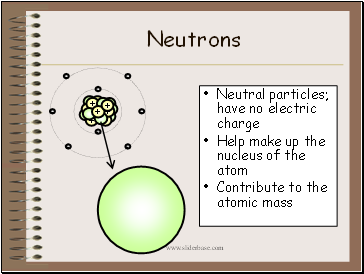
Neutrons
Neutral particles; have no electric charge
Help make up the nucleus of the atom
Contribute to the atomic mass
Slide 9
Electrons (-)
Negatively charged particles
Found outside the nucleus of the atom, in the electron orbits/levels; each orbit/level can hold a maximum number of electrons ( 1st = 2, 2nd = 8, 3rd = 8 or 18, etc…)
Move so rapidly around the nucleus that they create an electron cloud
Mass is insignificant when compared to protons and neutrons
Equal to the number of protons
Involved in the formation of chemical bonds
-
Slide 10
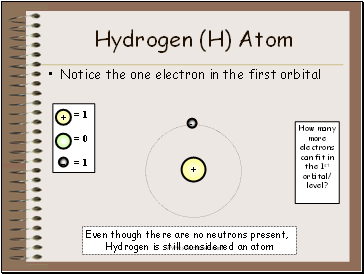
Hydrogen (H) Atom
Notice the one electron in the first orbital
+
-
Even though there are no neutrons present,
Hydrogen is still considered an atom
How many
more electrons
can fit in the 1st
orbital/ level?
Slide 11
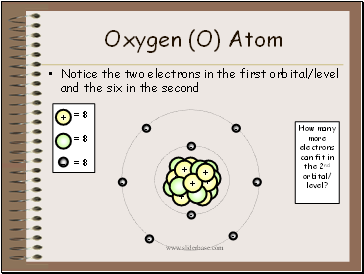
Oxygen (O) Atom
Notice the two electrons in the first orbital/level and the six in the second
+
+
+
+
-
-
-
-
-
-
-
-
+
How many
more electrons
can fit in the 2nd
orbital/ level?
Slide 12
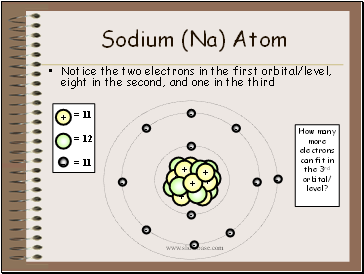
Sodium (Na) Atom
Notice the two electrons in the first orbital/level, eight in the second, and one in the third
+
+
+
+
-
-
-
-
-
-
-
-
+
-
-
-
How many
more electrons
can fit in the 3rd
orbital/ level?
Slide 13
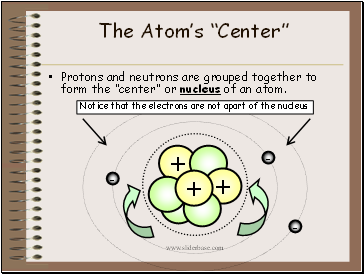
The Atom’s “Center”
Protons and neutrons are grouped together to form the “center” or nucleus of an atom.
-
Notice that the electrons are not apart of the nucleus
-
-
Slide 14
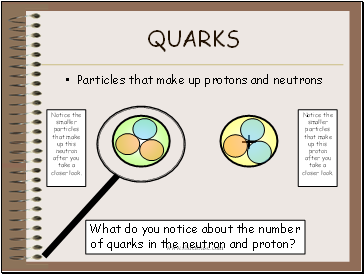
Quarks
Particles that make up protons and neutrons
Notice the smaller particles that make up this neutron after you take a closer look.
+
Notice the smaller particles that make up this proton after you take a closer look.
Contents
- Matter
- Atoms
- Atoms are so small that…
- Let’s Experiment
- Results
- Protons (+)
- Neutrons
- Electrons (-)
- Hydrogen (H) Atom
- Oxygen (O) Atom
- Sodium (Na) Atom
- The Atom’s “Center”
- Quarks
- Atomic Number
- Mass Number
- Building Atoms
- Atom Builder
- Gravitational Force
- Electromagnetic Force
- Strong Force
- Weak Force
- Isotopes
- Atomic Mass
- Ion
- Building Ions
Last added presentations
- Solar Thermal Energy
- Space Radiation
- Thermal Energy
- Sensory and Motor Mechanisms
- Resource Acquisition and Transport in Vascular Plants
- Radioactivity and Nuclear Reactions
- Newton's laws of motion