The pH ScalePage
4
4
Rain with a pH below 5.6 is “acid rain“
CO2 in the air forms carbonic acid
CO2 + H2O H2CO3
Adds to H+ of rain
H2CO3 H+ (aq) + HCO3-(aq)
Formation of acid rain:
1. Emission of sulfur and nitrogen oxides from the burning of fuels expecially coal with high S content, power stations, oil refineries, vehicles as well as bacterial decomposition, and lighting hitting N2
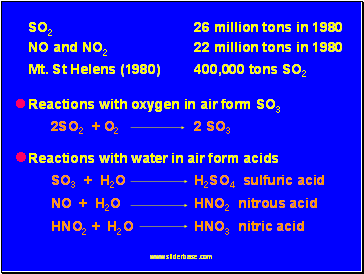
SO2 26 million tons in 1980
NO and NO2 22 million tons in 1980
Mt. St Helens (1980) 400,000 tons SO2
2. Reactions in the atmosphere form SO3
2SO2 + O2 2 SO3
3. Reactions with atmosphere water form acids
SO3 + H2O H2SO4 sulfuric acid
NO + H2O HNO2 nitrous acid
HNO2 + H2O HNO3 nitric acid
4. Effects of Acid Rain
Decline in fish populations in rivers and lasts due to toxic effect of Al leached from soil by acid rain
Extensive fish kills in spring from runoff due to accumulation of large amounts of acid on the snow
Dissolves minerals Mg, Ca, and K from the soil and waxy coatings that protect leaves from bacteria
Corrodes metals, textiles, paper and leather
Slide 31
Sources of Acid Rain
LecturePLUS Timberlake
31
Power stations
Oil refineries
Coal with high S content
Car and truck emissions
Bacterial decomposition, and lighting hitting N2
Slide 32
LecturePLUS Timberlake
32
SO2 26 million tons in 1980
NO and NO2 22 million tons in 1980
Mt. St Helens (1980) 400,000 tons SO2
Reactions with oxygen in air form SO3
2SO2 + O2 2 SO3
Reactions with water in air form acids
SO3 + H2O H2SO4 sulfuric acid
NO + H2O HNO2 nitrous acid
HNO2 + H2O HNO3 nitric acid
Slide 33
Effects of Acid Rain
LecturePLUS Timberlake
33
Leaches Al from soil, which kills fish
Fish kills in spring from runoff due to accumulation of large amounts of acid in snow
Dissolves waxy coatings that protect leaves from bacteria
Corrodes metals, textiles, paper and leather
Contents
- Ionization of Water
- Pure Water is Neutral
- Ion Product of Water Kw
- Acids
- Bases
- Using the Calculator
- Acid Rain
- Sources of Acid Rain
- Effects of Acid Rain
Last added presentations
- Solar Thermal Energy
- Friction
- Health Physics
- Ch 9 Nuclear Radiation
- Radiation Safety and Operations
- Newton's laws of motion
- Magnetic field uses sound waves to ignite sun's ring of fire