Carbon and the Molecular Diversity of LifePage
1
1
Slide 1
Carbon: The Backbone of Life
Although cells are 70–95% water, the rest consists mostly of carbon-based compounds
Carbon is unparalleled in its ability to form large, complex, and diverse molecules
Proteins, DNA, carbohydrates, and other molecules that distinguish living matter are all composed of carbon compounds
Slide 2
Fig. 4-1
Slide 3
Concept 4.1: Organic chemistry is the study of carbon compounds
Organic chemistry is the study of compounds that contain carbon
Organic compounds range from simple molecules to colossal ones
Most organic compounds contain hydrogen atoms in addition to carbon atoms
Slide 4
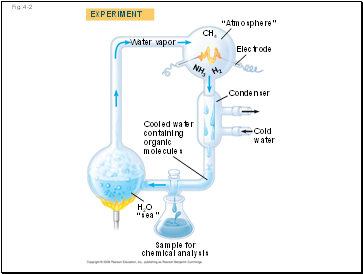
Vitalism, the idea that organic compounds arise only in organisms, was disproved when chemists synthesized these compounds
Mechanism is the view that all natural phenomena are governed by physical and chemical laws
Slide 5
Fig. 4-2
Water vapor
H2
NH3
“Atmosphere”
Electrode
Condenser
Cold
water
Cooled water
containing
organic
molecules
Sample for
chemical analysis
H2O
“sea”
EXPERIMENT
CH4
Slide 6
Concept 4.2: Carbon atoms can form diverse molecules by bonding to four other atoms
Electron configuration is the key to an atom’s characteristics
Electron configuration determines the kinds and number of bonds an atom will form with other atoms
Slide 7
The Formation of Bonds with Carbon
With four valence electrons, carbon can form four covalent bonds with a variety of atoms
This tetravalence makes large, complex molecules possible
In molecules with multiple carbons, each carbon bonded to four other atoms has a tetrahedral shape
However, when two carbon atoms are joined by a double bond, the molecule has a flat shape
Slide 8
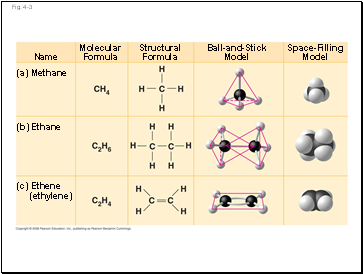
Fig. 4-3
Name
Molecular Formula
Structural Formula
Ball-and-Stick
Model
Space-Filling
Model
(a) Methane
(b) Ethane
(c) Ethene
(ethylene)
Slide 9
The electron configuration of carbon gives it covalent compatibility with many different elements
The valences of carbon and its most frequent partners (hydrogen, oxygen, and nitrogen) are the “building code” that governs the architecture of living molecules
Contents
- Carbon: The Backbone of Life
- The Formation of Bonds with Carbon
- Molecular Diversity Arising from Carbon Skeleton Variation
- Hydrocarbons
- Isomers
- The Chemical Groups Most Important in the Processes of Life
- ATP: An Important Source of Energy for Cellular Processes
- The Chemical Elements of Life: A Review
Last added presentations
- Newton's Laws
- Solar Energy
- Newton’s Law of Gravity
- Solar Thermal Energy
- Newton’s third law of motion
- Newton’s Laws of Motion
- Static and Kinetic Friction