PeriodicTablePage
1
1
Slide 1
Periodic Table of the Elements
Slide 2
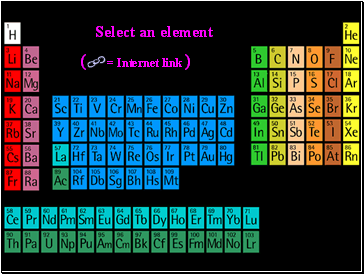
Select an element
= Internet link
(
)
Slide 3
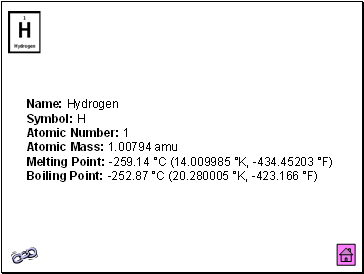
Hydrogen
Name: Hydrogen Symbol: H Atomic Number: 1 Atomic Mass: 1.00794 amu Melting Point: -259.14 °C (14.009985 °K, -434.45203 °F) Boiling Point: -252.87 °C (20.280005 °K, -423.166 °F)
Slide 4
Helium
Name: Helium Symbol: He Atomic Number: 2 Atomic Mass: 4.002602 amu Melting Point: -272.0 °C (1.15 °K, -457.6 °F) Boiling Point: -268.6 °C (4.549994 °K, -451.48 °F)
Slide 5
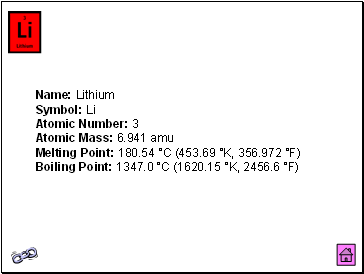
Lithium
Name: Lithium Symbol: Li Atomic Number: 3 Atomic Mass: 6.941 amu Melting Point: 180.54 °C (453.69 °K, 356.972 °F) Boiling Point: 1347.0 °C (1620.15 °K, 2456.6 °F)
Slide 6
Beryllium
Name: Beryllium Symbol: Be Atomic Number: 4 Atomic Mass: 9.012182 amu Melting Point: 1278.0 °C (1551.15 °K, 2332.4 °F) Boiling Point: 2970.0 °C (3243.15 °K, 5378.0 °F)
Slide 7
Boron
Name: Boron Symbol: B Atomic Number: 5 Atomic Mass: 10.811 amu Melting Point: 2300.0 °C (2573.15 °K, 4172.0 °F) Boiling Point: 2550.0 °C (2823.15 °K, 4622.0 °F)
Slide 8
Carbon
Name: Carbon Symbol: C Atomic Number: 6 Atomic Mass: 12.0107 amu Melting Point: 3500.0 °C (3773.15 °K, 6332.0 °F) Boiling Point: 4827.0 °C (5100.15 °K, 8720.6 °F)
Slide 9
Nitrogen
Name: Nitrogen Symbol: N Atomic Number: 7 Atomic Mass: 14.00674 amu Melting Point: -209.9 °C (63.250008 °K, -345.81998 °F) Boiling Point: -195.8 °C (77.35 °K, -320.44 °F)
Slide 10
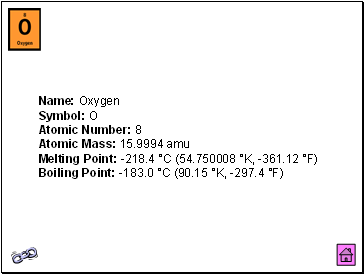
Oxygen
Name: Oxygen Symbol: O Atomic Number: 8 Atomic Mass: 15.9994 amu Melting Point: -218.4 °C (54.750008 °K, -361.12 °F) Boiling Point: -183.0 °C (90.15 °K, -297.4 °F)
Slide 11
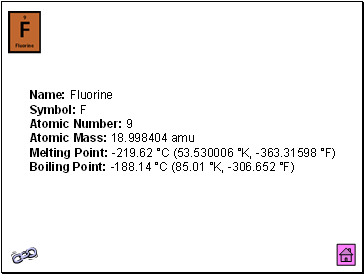
Fluorine
Name: Fluorine Symbol: F Atomic Number: 9 Atomic Mass: 18.998404 amu Melting Point: -219.62 °C (53.530006 °K, -363.31598 °F) Boiling Point: -188.14 °C (85.01 °K, -306.652 °F)
Slide 12
Neon
Contents
- Periodic Table of the Elements
- Hydrogen
- Helium
- Lithium
- Beryllium
- Boron
- Carbon
- Nitrogen
- Oxygen
- Fluorine
- Neon
- Sodium
- Magnesium
- Aluminum
- Silicon
- Phosphorus
- Sulfur
- Chlorine
- Argon
- Potassium
- Calcium
- Scandium
- Titanium
- Vanadium
- Chromium
- Manganese
- Iron
- Cobalt
- Nickel
- Copper
- Zinc
- Gallium
- Germanium
- Arsenic
- Selenium
- Bromine
- Krypton
- Rubidium
- Strontium
- Yttrium
- Zirconium
- Niobium
- Molybdenum
- Technetium
- Ruthenium
- Rhodium
- Palladium
- Silver
- Cadmium
- Indium
- Tin
- Antimony
- Tellurium
- Iodine
- Xenon
- Cesium
- Barium
- Lanthanum
- Cerium
- Praseodymium
- Neodymium
- Promethium
- Samarium
- Europium
- Gadolinium
- Terbium
- Dysprosium
- Holmium
- Erbium
- Thulium
- Ytterbium
- Lutetium
- Hafnium
- Tantalum
- Tungsten
- Rhenium
- Osmium
- Iridium
- Platinum
- Gold
- Mercury
- Thallium
- Lead
- Bismuth
- Polonium
- Astatine
- Radon
- Francium
- Radium
- Actinium
- Thorium
- Protactinium
- Uranium
- Neptunium
- Plutonium
- Americium
- Curium
- Berkelium
- Californium
- Einsteinium
- Fermium
- Mendelevium
- Nobelium
- Lawrencium
- Rutherfordium
- Dubnium
- Seaborgium
- Bohrium
- Hassium
- Meitnerium
Last added presentations
- Radioactivity and Nuclear Reactions
- Practical Applications of Solar Energy
- Resource Acquisition and Transport in Vascular Plants
- Newton’s law of universal gravitation
- Newton’s laws of motion
- Friction
- Static and Kinetic Friction