Properties of SolidsPage
2
2
steel = iron + carbon
Slide 14
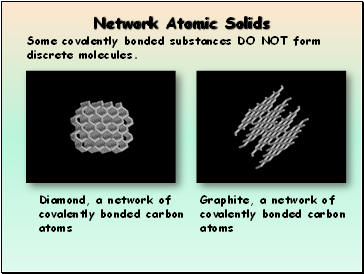
Network Atomic Solids
Some covalently bonded substances DO NOT form discrete molecules.
Diamond, a network of covalently bonded carbon atoms
Graphite, a network of covalently bonded carbon atoms
Slide 15
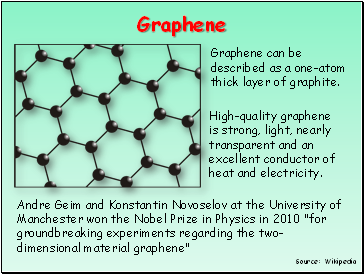
Graphene
Graphene can be described as a one-atom thick layer of graphite.
Source: Wikipedia
High-quality graphene is strong, light, nearly transparent and an excellent conductor of heat and electricity.
Andre Geim and Konstantin Novoselov at the University of Manchester won the Nobel Prize in Physics in 2010 "for groundbreaking experiments regarding the two-dimensional material graphene"
Slide 16
Semiconductors
Pure silicon is structurally the same as diamond, but is a semiconductor rather than an insulator.
The conductivity increases at higher temperature.
Conductivity of silicon can be improved by doping with other elements.
Slide 17
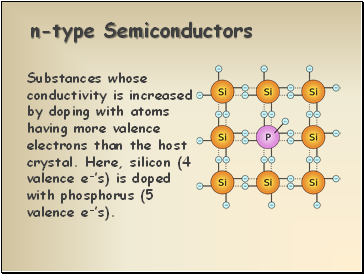
n-type Semiconductors
Substances whose conductivity is increased by doping with atoms having more valence electrons than the host crystal. Here, silicon (4 valence e-’s) is doped with phosphorus (5 valence e-’s).
Slide 18
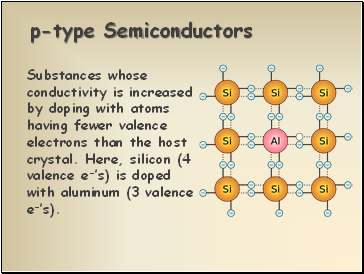
p-type Semiconductors
Substances whose conductivity is increased by doping with atoms having fewer valence electrons than the host crystal. Here, silicon (4 valence e-’s) is doped with aluminum (3 valence e-’s).
Slide 19
Molecular Solids
Strong covalent forces within molecules
Weak covalent forces between molecules
Sulfur, S8
Phosphorus, P4
Slide 20
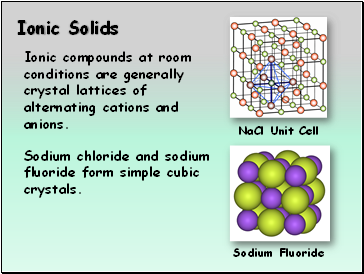
Ionic Solids
Sodium Fluoride
Ionic compounds at room conditions are generally crystal lattices of alternating cations and anions.
Sodium chloride and sodium fluoride form simple cubic crystals.
NaCl Unit Cell
Slide 21
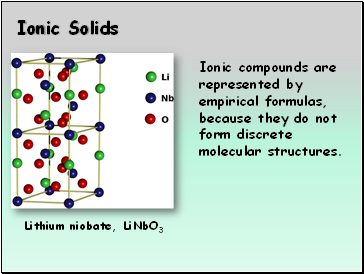
Ionic Solids
Lithium niobate, LiNbO3
Ionic compounds are represented by empirical formulas, because they do not form discrete molecular structures.
1 2
Contents
- Types of Solids
- Representation of Components in a Crystalline Solid
- Bragg’s Law
- Crystal Structures - Cubic
- Crystal Structures - Monoclinic
- Crystal Structures - Tetragonal
- Crystal Structures - Orthorhombic
- Crystal Structures – Other Shapes
- Closest Packing: Single Layer
- Closest Packing: Multiple Layers
- Metal Alloys
- Network Atomic Solids
- Graphene
- Semiconductors
- n-type Semiconductors
- p-type Semiconductors
- Molecular Solids
- Ionic Solids
Last added presentations
- Heat-Energy on the Move
- Sensory and Motor Mechanisms
- History of Modern Astronomy
- Waves & Sound
- Friction
- Direct heat utilization of geothermal energy
- Geophysical Concepts, Applications and Limitations