Rate LawsPage
2
2
2
+
1
= 3
The reaction is 3rd order
Slide 11
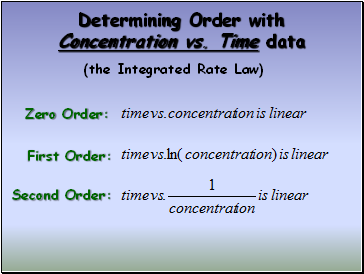
Determining Order with Concentration vs. Time data
(the Integrated Rate Law)
Zero Order:
First Order:
Second Order:
Slide 12
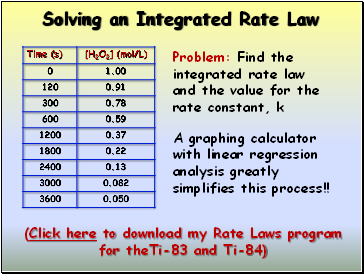
Solving an Integrated Rate Law
Problem: Find the integrated rate law and the value for the rate constant, k
A graphing calculator with linear regression analysis greatly simplifies this process!!
(Click here to download my Rate Laws program for theTi-83 and Ti-84)
Slide 13
Time vs. [H2O2]
y = ax + b
a = -2.64 x 10-4
b = 0.841
r2 = 0.8891
r = -0.9429
Regression results:
Slide 14
Time vs. ln[H2O2]
Regression results:
y = ax + b
a = -8.35 x 10-4
b = -.005
r2 = 0.99978
r = -0.9999
Slide 15
Time vs. 1/[H2O2]
y = ax + b
a = 0.00460
b = -0.847
r2 = 0.8723
r = 0.9340
Regression results:
Slide 16
And the winner is… Time vs. ln[H2O2]
1. As a result, the reaction is 1st order
2. The (differential) rate law is:
3. The integrated rate law is:
4. But…what is the rate constant, k ?
Slide 17
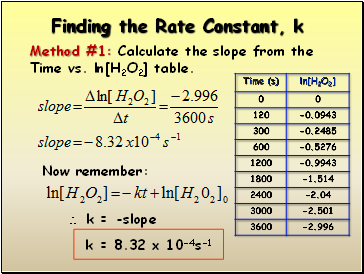
Finding the Rate Constant, k
Method #1: Calculate the slope from the
Time vs. ln[H2O2] table.
Now remember:
k = -slope
k = 8.32 x 10-4s-1
Slide 18
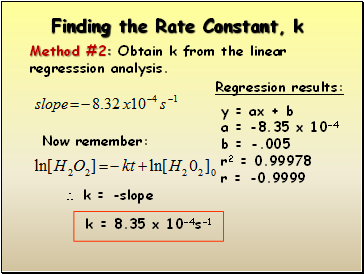
Finding the Rate Constant, k
Method #2: Obtain k from the linear regresssion analysis.
Now remember:
k = -slope
k = 8.35 x 10-4s-1
Regression results:
y = ax + b
a = -8.35 x 10-4
b = -.005
r2 = 0.99978
r = -0.9999
Slide 19
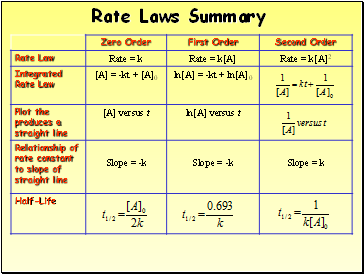
Rate Laws Summary
1 2
Contents
- Reaction Rate
- Rate Laws
- Determining Order with Concentration vs. Time data
- Solving an Integrated Rate Law
- Finding the Rate Constant, k
- Rate Laws Summary
Last added presentations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Radiation Safety and Operations
- Resource Acquisition and Transport in Vascular Plants
- Madame Marie Curie
- Thermal Energy
- Radiation
- The Effects of Radiation on Living Things

![Time vs. [H2O2] Time vs. [H2O2]](images/referats/167/image013.png)
![Time vs. ln[H2O2] Time vs. ln[H2O2]](images/referats/167/image014.png)
![Time vs. 1/[H2O2] Time vs. 1/[H2O2]](images/referats/167/image015.png)
![And the winner is… Time vs. ln[H2O2] And the winner is… Time vs. ln[H2O2]](images/referats/167/image016.png)