The d BlockPage
1
1
Slide 1
The d block
The d block consists of three horizontal series in periods 4, 5 & 6
10 elements in each series
Chemistry is ďdifferentĒ from other elements
Special electronic configurations important
Differences within a group in the d block are less sharp than in s & p block
Similarities across a period are greater
Slide 2
Electronic Configuration
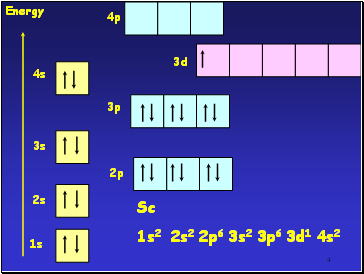
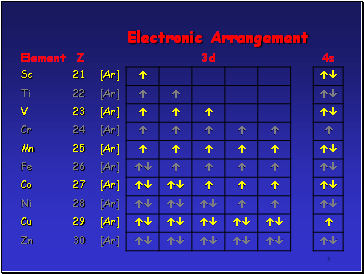
Across the 1st row of the d block (Sc to Zn) each element
has 1 more electron and 1 more proton
Each ďadditionalĒ electron enters the 3d sub-shell
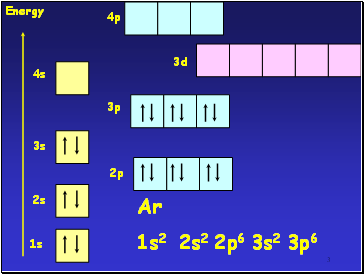
The core configuration for all the period 4 transition elements is that of Ar
1s22s22p63s23p6
Slide 3
3
1s
2s
3s
4s
2p
3p
3d
Energy
Ar
1s2 2s2 2p6 3s2 3p6
4p
Slide 4
4
1s
2s
3s
4s
2p
3p
3d
Energy
Sc
1s2 2s2 2p6 3s2 3p6 3d1 4s2
4p
Slide 5
5
Slide 6
Chromium and Copper
Cr and Cu donít fit the pattern of building up the 3d sub-shell, why?
In the ground state electrons are always arranged to give lowest total energy
Electrons are negatively charged and repel each other
Lower total energy is obtained with e- singly in orbitals rather than if they are paired in an orbital
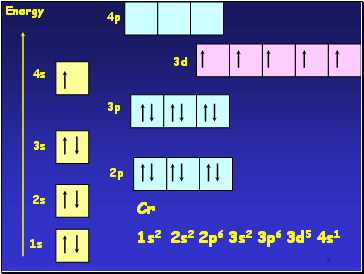
Energies of 3d and 4s orbitals very close together in Period 4
Slide 7
Chromium and Copper
At Cr
Orbital energies such that putting one e- into each 3d and 4s orbital gives lower energy than having 2 e- in the 4s orbital
At Cu
Putting 2 e- into the 4s orbital would give a higher energy than filling the 3d orbitals
Slide 8
8
1s
2s
3s
4s
2p
3p
3d
Energy
Cr
1s2 2s2 2p6 3s2 3p6 3d5 4s1
4p
Slide 9
9
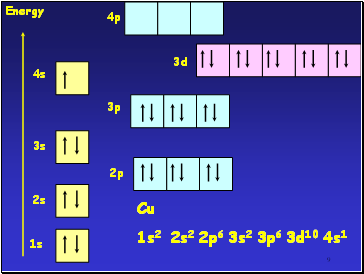
1s
2s
3s
4s
2p
3p
3d
Energy
Cu
1s2 2s2 2p6 3s2 3p6 3d10 4s1
4p
Slide 10
What is a transition metal?
Transition metals [TMís] have characteristic properties
e.g. coloured compounds, variable oxidation states
These are due to presence of an inner incomplete d sub-shell
Contents
- The d block
- Electronic Configuration
- Chromium and Copper
- What is a transition metal?
- What are TMís like?
- Effect of Alloying on TMís
- TM Chemical Properties
- Variable Oxidation States
- Oxidation States of TMís
- Stability of OSís
- Catalytic Activity
- Heterogeneous Catalysis
- Suggested Mechanism
Last added presentations
- Mechanics Lecture
- Resource Acquisition and Transport in Vascular Plants
- The Effects of Radiation on Living Things
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Radiation Safety and Operations
- Newton's Laws
- Sound