The d BlockPage
4
4
Slide 27
Catalytic Activity
TMís and their compounds effective and important catalysts
Industrially and biologically!!
The ďpeople in the knowĒ believe
catalysts provide reaction pathway with lower EA than uncatalysed reaction (see CI 10.5)
Once again,
availability of 3d and 4s e-
ability to change OS
among factors which make TMís such good catalysts
Slide 28
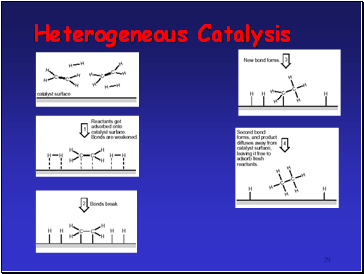
Heterogeneous Catalysis
Catalyst in different phase from reactants
Usually means solid TM catalyst with reactants in liquid or gas phases
TMís can
use the 3d and 4s e- of atoms on metal surface to from weak bonds to the reactants.
Once reaction has occurred on TM surface, these bonds can break to release products
Important example is hydrogenation of alkenes using Ni or Pt catalyst
Slide 29
Heterogeneous Catalysis
Slide 30
Homogeneous Catalysis
Catalyst in same phase as reactants
Usually means reaction takes place in aqueous phase
Catalyst aqueous TM ion
Usually involves
TM ion forming intermediate compound with ome or more of the reactants
Intermediate then breaks down to form products
Slide 31
31
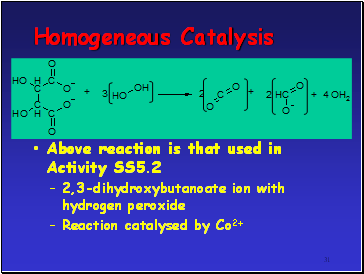
Homogeneous Catalysis
Above reaction is that used in Activity SS5.2
2,3-dihydroxybutanoate ion with hydrogen peroxide
Reaction catalysed by Co2+
Slide 32
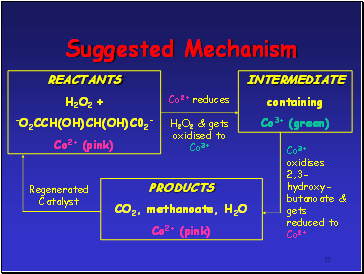
Suggested Mechanism
REACTANTS
H2O2 +
-O2CCH(OH)CH(OH)C02-
Co2+ (pink)
INTERMEDIATE
containing
Co3+ (green)
PRODUCTS
CO2, methanoate, H2O
Co2+ (pink)
Contents
- The d block
- Electronic Configuration
- Chromium and Copper
- What is a transition metal?
- What are TMís like?
- Effect of Alloying on TMís
- TM Chemical Properties
- Variable Oxidation States
- Oxidation States of TMís
- Stability of OSís
- Catalytic Activity
- Heterogeneous Catalysis
- Suggested Mechanism
Last added presentations
- Heat-Energy on the Move
- Mechanics Lecture
- Thermal Energy
- Simulation at NASA for the Space Radiation Effort
- Magnetic field uses sound waves to ignite sun's ring of fire
- Gravitation
- Resource Acquisition and Transport in Vascular Plants