Atomic Structure, History of the AtomPage
2
2
Slide 10
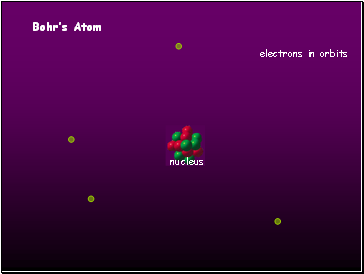
Bohrís Atom
electrons in orbits
nucleus
Slide 11
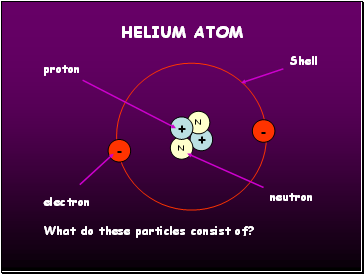
Helium atom
+
N
N
+
-
-
proton
electron
neutron
Shell
What do these particles consist of?
Slide 12
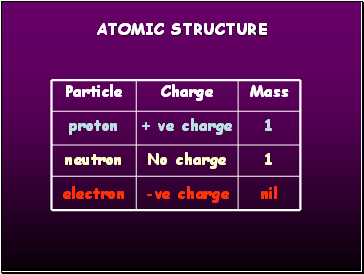
Atomic structure
Particle
proton
neutron
electron
Charge
+ ve charge
-ve charge
No charge
1
1
nil
Mass
Slide 13
ATOMIC STRUCTURE
the number of protons in an atom
the number of protons and
neutrons in an atom
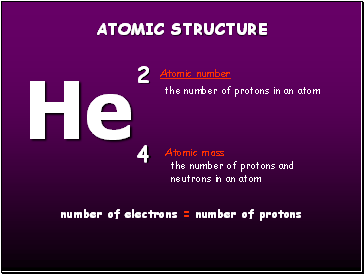
He
2
4
Atomic mass
Atomic number
number of electrons = number of protons
Slide 14
ATOMIC STRUCTURE
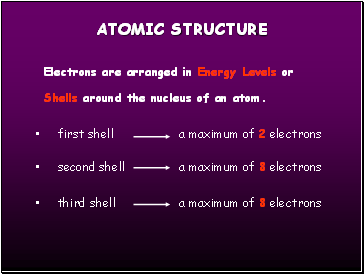
Electrons are arranged in Energy Levels or Shells around the nucleus of an atom.
first shell a maximum of 2 electrons
second shell a maximum of 8 electrons
third shell a maximum of 8 electrons
Slide 15
ATOMIC STRUCTURE
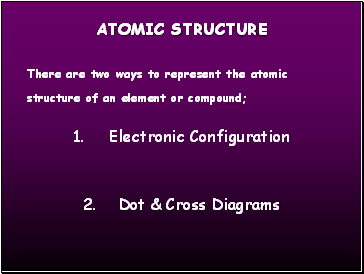
There are two ways to represent the atomic structure of an element or compound;
1. Electronic Configuration
2. Dot & Cross Diagrams
Slide 16
Electronic configuration
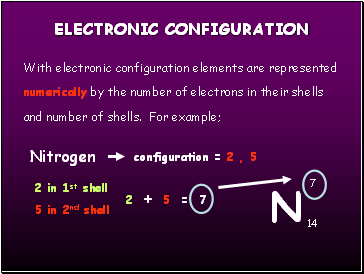
With electronic configuration elements are represented numerically by the number of electrons in their shells and number of shells. For example;
N
Nitrogen
7
14
2 in 1st shell
5 in 2nd shell
configuration = 2 , 5
2 + 5 = 7
Slide 17
ELECTRONIC CONFIGURATION
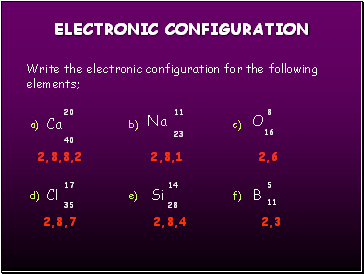
Write the electronic configuration for the following elements;
Ca
O
Cl
Si
Na
20
40
11
23
8
17
16
35
14
28
B
11
5
a)
b)
c)
d)
e)
f)
2,8,8,2
2,8,1
2,8,7
2,8,4
2,3
2,6
Slide 18
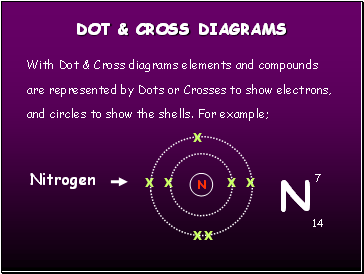
Dot & Cross diagrams
With Dot & Cross diagrams elements and compounds are represented by Dots or Crosses to show electrons, and circles to show the shells. For example;
Nitrogen
N
X
X
X
X
X
X
X
N
7
14
Slide 19
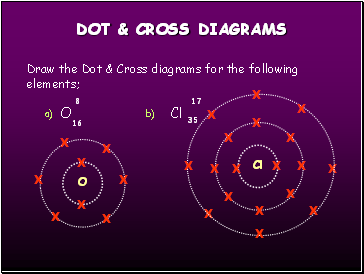
DOT & CROSS DIAGRAMS
Draw the Dot & Cross diagrams for the following elements;
O
Cl
8
17
16
35
a)
Contents
- Atomic Structure
- History of the atom
- Bohrís Atom
- Helium atom
- Atomic structure
- Electronic configuration
- Dot & Cross diagrams
- Summary
Last added presentations
- History of Modern Astronomy
- Magnetic field uses sound waves to ignite sun's ring of fire
- The Effects of Radiation on Living Things
- Newtonís law of universal gravitation
- Radiation Safety and Operations
- Newtonís third law of motion
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal