AtomsPage
1
1
Slide 1
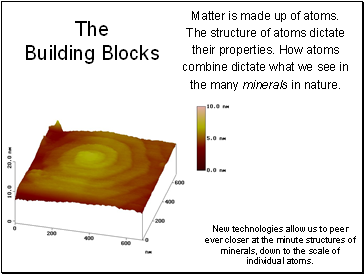
The Building Blocks
Matter is made up of atoms.
The structure of atoms dictate their properties. How atoms combine dictate what we see in the many minerals in nature.
New technologies allow us to peer ever closer at the minute structures of minerals, down to the scale of individual atoms.
Slide 2
The Stuff That Makes Atoms
Although one can subdivide atoms into numerous
subatomic particles, we will be concerned only with
protons, neutrons and electrons.
Protons and neutrons are together in the nucleus of an atom, whereas electrons are in motion in orbits
around the central nucleus.
Protons carry a positive electrical charge, electrons carry a negative charge, and neutrons carry no charge.
Neutrons work to keep nuclei together.
Most atoms are electrically neutral, meaning that they have an equal number of protons and electrons.
Slide 3
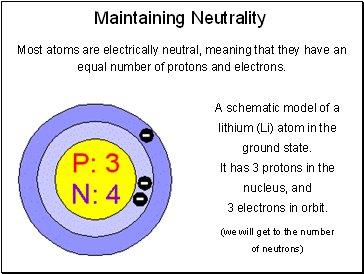
Maintaining Neutrality
Most atoms are electrically neutral, meaning that they have an equal number of protons and electrons.
A schematic model of a lithium (Li) atom in the ground state.
It has 3 protons in the nucleus, and
3 electrons in orbit.
(we will get to the number
of neutrons)
Slide 4
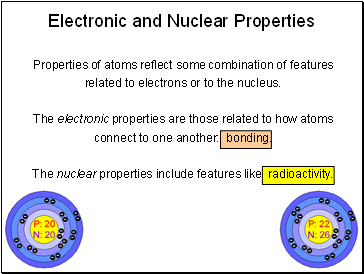
Electronic and Nuclear Properties
Properties of atoms reflect some combination of features related to electrons or to the nucleus.
The electronic properties are those related to how atoms connect to one another: bonding.
The nuclear properties include features like radioactivity.
Slide 5
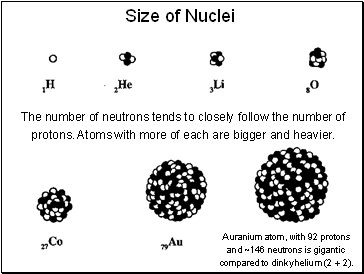
Size of Nuclei
The number of neutrons tends to closely follow the number of protons. Atoms with more of each are bigger and heavier.
A uranium atom, with 92 protons and ~146 neutrons is gigantic compared to dinky helium (2 + 2).
Slide 6
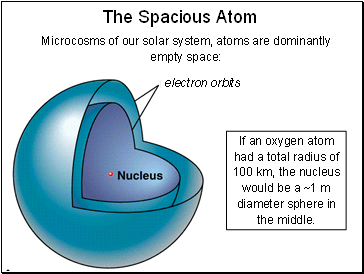
The Spacious Atom
Microcosms of our solar system, atoms are dominantly empty space:
If an oxygen atom had a total radius of 100 km, the nucleus would be a ~1 m diameter sphere in the middle.
electron orbits
Slide 7
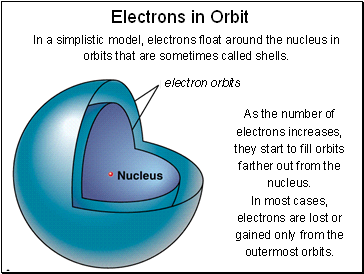
Electrons in Orbit
In a simplistic model, electrons float around the nucleus in orbits that are sometimes called shells.
As the number of electrons increases, they start to fill orbits farther out from the nucleus.
In most cases, electrons are lost or gained only from the outermost orbits.
Contents
- The Building Blocks
- The Stuff That Makes Atoms
- Maintaining Neutrality
- Electronic and Nuclear Properties
- Size of Nuclei
- The Spacious Atom
- Electrons in Orbit
- Charged Atoms: Ions
- Atomic Number
- The Periodic Table
- Oxidation State
- Oxidized and Reduced States
- The Periodic Table: the Bulk Earth
- The Periodic Table: the Crust
- Atomic Weight: It’s all in the Nucleus
- Isotopes
- Radioactive or Stable?
- Stable and Radioactive Isotopes
- Radioactivity Inside You
- Chemical Bonds
- Ionic Bonds
- Covalent Bonds: Electron Sharing
- Alternative Bonds
- Chemical Reactions: Achieving Stability
- What is a Mineral?
- Stability
- Stability and Metastability
Last added presentations
- Thermal Energy
- Madame Marie Curie
- Motion
- Newton’s law of universal gravitation
- Buoyancy
- History of Modern Astronomy
- Newton’s Laws of Motion