Earth MaterialsPage
5
5
Slide 35
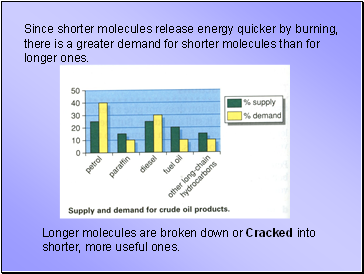
Since shorter molecules release energy quicker by burning, there is a greater demand for shorter molecules than for longer ones.
Longer molecules are broken down or Cracked into shorter, more useful ones.
Slide 36
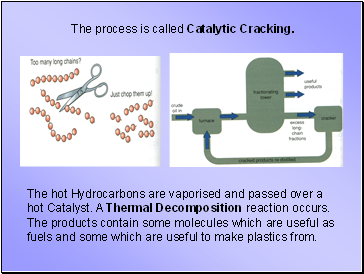
The process is called Catalytic Cracking.
The hot Hydrocarbons are vaporised and passed over a hot Catalyst. A Thermal Decomposition reaction occurs. The products contain some molecules which are useful as fuels and some which are useful to make plastics from.
Slide 37
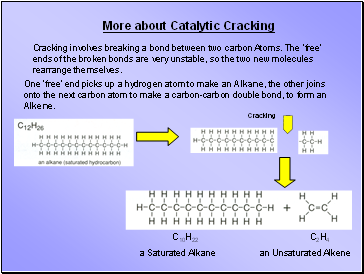
More about Catalytic Cracking
Cracking involves breaking a bond between two carbon Atoms. The ‘free’ ends of the broken bonds are very unstable, so the two new molecules rearrange themselves.
One ‘free’ end picks up a hydrogen atom to make an Alkane, the other joins onto the next carbon atom to make a carbon-carbon double bond, to form an Alkene.
C10H22
a Saturated Alkane
C2H4
an Unsaturated Alkene
Slide 38
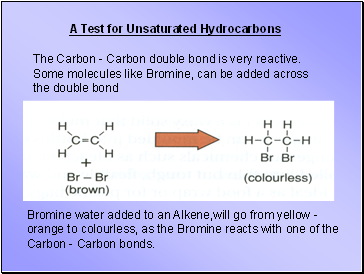
A Test for Unsaturated Hydrocarbons
The Carbon - Carbon double bond is very reactive. Some molecules like Bromine, can be added across the double bond
Bromine water added to an Alkene,will go from yellow - orange to colourless, as the Bromine reacts with one of the Carbon - Carbon bonds.
Slide 39
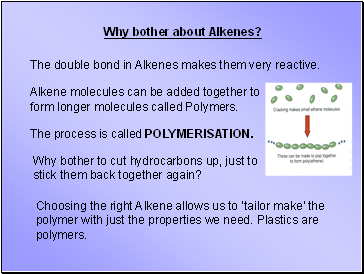
Why bother about Alkenes?
The double bond in Alkenes makes them very reactive.
Alkene molecules can be added together to form longer molecules called Polymers.
The process is called POLYMERISATION.
Why bother to cut hydrocarbons up, just to stick them back together again?
Choosing the right Alkene allows us to ‘tailor make’ the polymer with just the properties we need. Plastics are polymers.
Slide 40
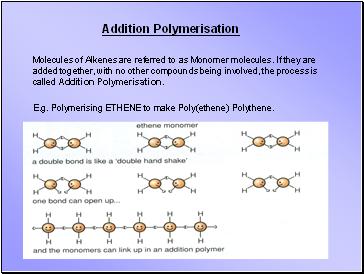
Addition Polymerisation
Molecules of Alkenes are referred to as Monomer molecules. If they are added together, with no other compounds being involved, the process is called Addition Polymerisation.
E.g. Polymerising ETHENE to make Poly(ethene) Polythene.
Slide 41
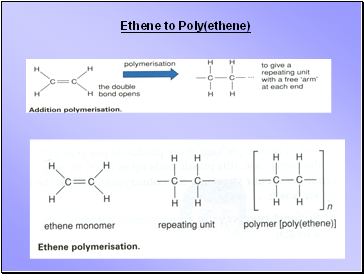
Ethene to Poly(ethene)
Slide 42
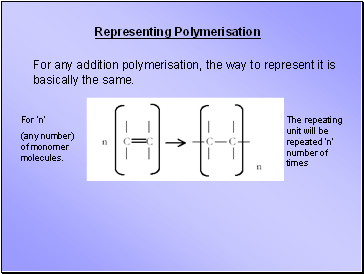
Representing Polymerisation
For any addition polymerisation, the way to represent it is basically the same.
For ‘n’
(any number) of monomer molecules.
The repeating unit will be repeated ‘n’ number of times
Slide 43
Contents
- Earth Materials. Module 06
- Limestone
- Reminder:
- Cement
- Glass
- The Oil is often found in porous rock
- Reminder!
- More about Catalytic Cracking
- A Test for Unsaturated Hydrocarbons
- Why bother about Alkenes?
- Addition Polymerisation
- Ethene to Poly(ethene)
- Representing Polymerisation
- Some uses of Plastics
- Problems with Plastics
- Why not recycle plastics?
- Why not burn them then?
- The need for a balanced solution
Last added presentations
- Motion
- Thermal Energy
- Newton’s Law of Gravity
- Soil and Plant Nutrition
- Buoyancy
- Static and Kinetic Friction
- Gravitation