Geometric IsomerismPage
1
1
Slide 1
Chemical Ideas 3.5
Geometric Isomerism
(different geometries)
Slide 2
Types of isomerism
Slide 3
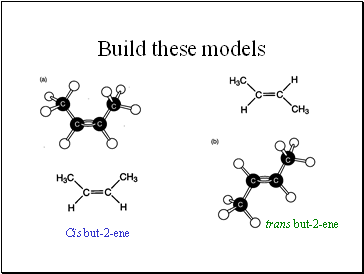
Build these models
Cis but-2-ene
trans but-2-ene
Slide 4
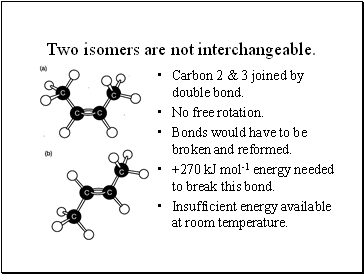
Two isomers are not interchangeable.
Carbon 2 & 3 joined by double bond.
No free rotation.
Bonds would have to be broken and reformed.
+270 kJ mol-1 energy needed to break this bond.
Insufficient energy available at room temperature.
Slide 5
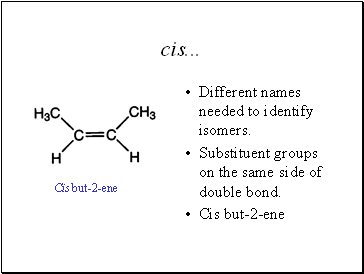
cis .
Different names needed to identify isomers.
Substituent groups on the same side of double bond.
Cis but-2-ene
Slide 6
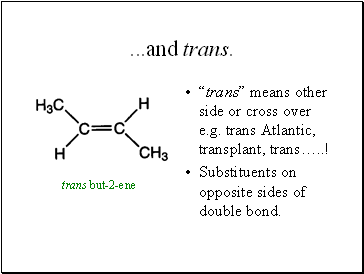
.and trans.
“trans” means other side or cross over e.g. trans Atlantic, transplant, trans… !
Substituents on opposite sides of double bond.
Slide 7
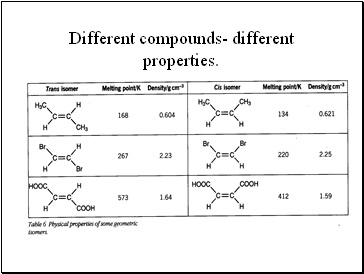
Different compounds- different properties.
Slide 8
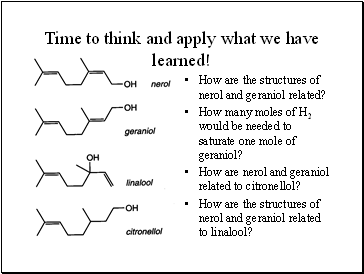
Time to think and apply what we have learned!
How are the structures of nerol and geraniol related?
How many moles of H2 would be needed to saturate one mole of geraniol?
How are nerol and geraniol related to citronellol?
How are the structures of nerol and geraniol related to linalool?
Contents
- Types of isomerism
- Build these models
- Two isomers are not interchangeable.
- Different compounds- different properties.
- Time to think and apply what we have learned!
Last added presentations
- Geophysical Concepts, Applications and Limitations
- The Effects of Radiation on Living Things
- Magnetic field uses sound waves to ignite sun's ring of fire
- Static and Kinetic Friction
- Newton’s law of universal gravitation
- Thermal Energy
- Newton’s Law of Gravity