Gas LawsPage
1
1
Slide 1
Gas Laws
Slide 2
CA Standards
Students know how to apply the gas laws to relations between the pressure, temperature, and volume of any amount of an ideal gas or any mixture of ideal gases.
Slide 3
Ideal Gases
Ideal gases are imaginary gases that perfectly fit all of the assumptions of the kinetic molecular theory.
Gases consist of tiny particles that are far apart relative to their size.
Collisions between gas particles and between particles and the walls of the container are elastic collisions
No kinetic energy is lost in elastic collisions
Slide 4
Ideal Gases (continued)
Gas particles are in constant, rapid motion. They therefore possess kinetic energy, the energy of motion
There are no forces of attraction between gas particles
The average kinetic energy of gas particles depends on temperature, not on the identity of the particle.
Slide 5
Real Gases Do Not Behave Ideally
Real gases DO experience inter-molecular attractions
Real gases DO have volume
Real gases DO NOT have elastic collisions
Slide 6
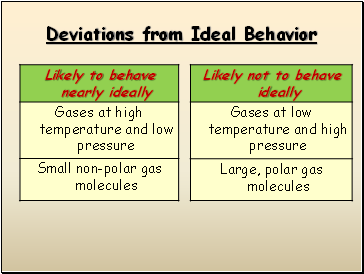
Deviations from Ideal Behavior
Slide 7
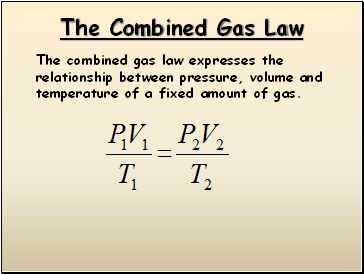
The Combined Gas Law
The combined gas law expresses the relationship between pressure, volume and temperature of a fixed amount of gas.
Slide 8
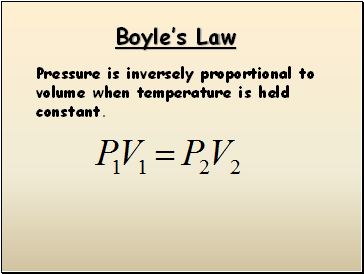
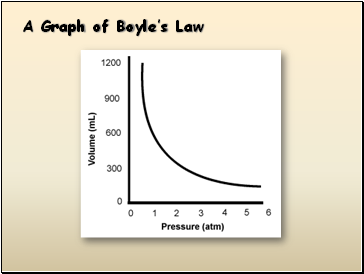
Boyle’s Law
Pressure is inversely proportional to volume when temperature is held constant.
Slide 9
A Graph of Boyle’s Law
Slide 10
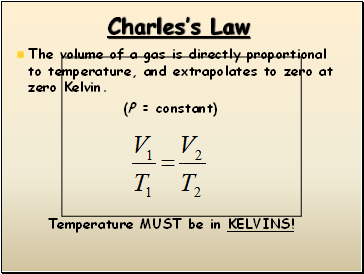
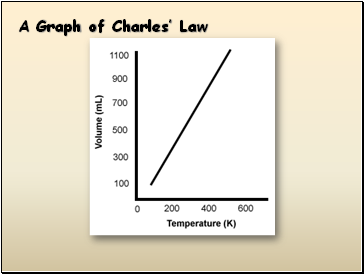
Charles’s Law
The volume of a gas is directly proportional to temperature, and extrapolates to zero at zero Kelvin.
(P = constant)
Temperature MUST be in KELVINS!
Slide 11
A Graph of Charles’ Law
Slide 12
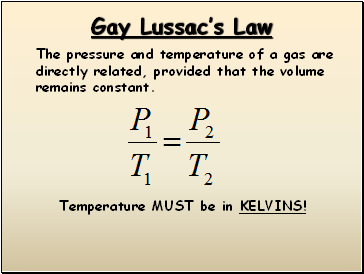
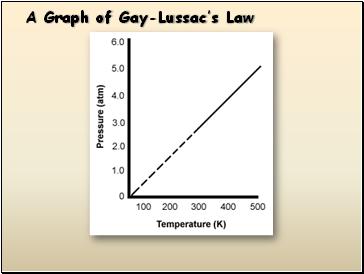
Gay Lussac’s Law
The pressure and temperature of a gas are
directly related, provided that the volume
remains constant.
Temperature MUST be in KELVINS!
Slide 13
1 2
Contents
- Gas Laws
- Ideal Gases
- Real Gases Do Not Behave Ideally
- Deviations from Ideal Behavior
- The Combined Gas Law
- Boyle’s Law
- A Graph of Boyle’s Law
- Charles’s Law
- A Graph of Charles’ Law
- Gay Lussac’s Law
- Dalton’s Law of Partial Pressures
Last added presentations
- Ch 9 Nuclear Radiation
- Solar Thermal Energy
- Practical Applications of Solar Energy
- Buoyancy
- Newton's laws of motion
- Static and Kinetic Friction
- Mechanics Lecture