Electrons and Wave-Particle DualityPage
1
1
Slide 1
The ELECTRON: Wave – Particle Duality
Graphic: www.lab-initio.com
Slide 2
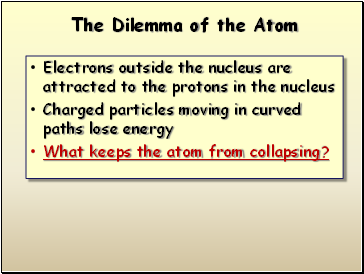
The Dilemma of the Atom
Electrons outside the nucleus are attracted to the protons in the nucleus
Charged particles moving in curved paths lose energy
What keeps the atom from collapsing?
Slide 3
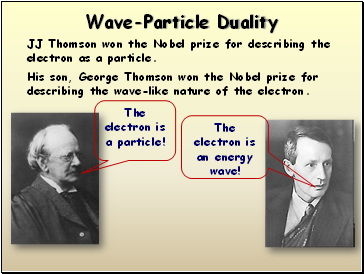
Wave-Particle Duality
JJ Thomson won the Nobel prize for describing the electron as a particle.
His son, George Thomson won the Nobel prize for describing the wave-like nature of the electron.
The electron is a particle!
The electron is an energy wave!
Slide 4
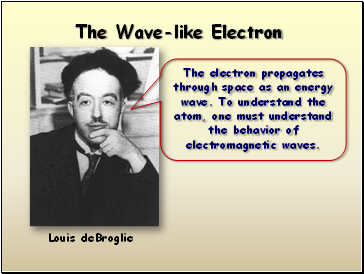
The Wave-like Electron
Louis deBroglie
The electron propagates through space as an energy wave. To understand the atom, one must understand the behavior of electromagnetic waves.
Slide 5
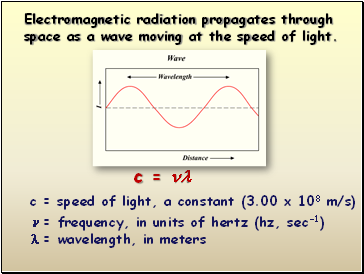
c =
c = speed of light, a constant (3.00 x 108 m/s)
= frequency, in units of hertz (hz, sec-1)
= wavelength, in meters
Electromagnetic radiation propagates through space as a wave moving at the speed of light.
Slide 6
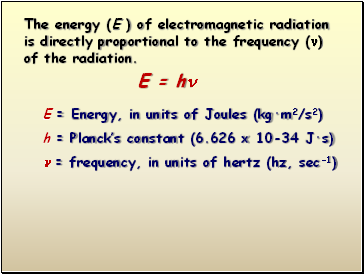
E = h
E = Energy, in units of Joules (kg·m2/s2)
h = Planck’s constant (6.626 x 10-34 J·s)
= frequency, in units of hertz (hz, sec-1)
The energy (E ) of electromagnetic radiation is directly proportional to the frequency () of the radiation.
Slide 7
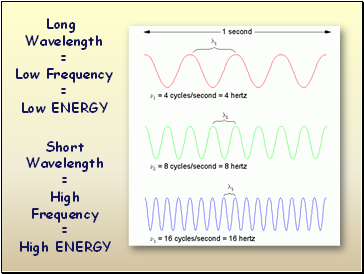
Long
Wavelength
=
Low Frequency
=
Low ENERGY
Short
Wavelength
=
High Frequency
=
High ENERGY
Wavelength Table
Slide 8
Answering the Dilemma of the Atom
Treat electrons as waves
As the electron moves toward the nucleus, the wavelength shortens
Shorter wavelength = higher energy
Higher energy = greater distance from the nucleus
Slide 9
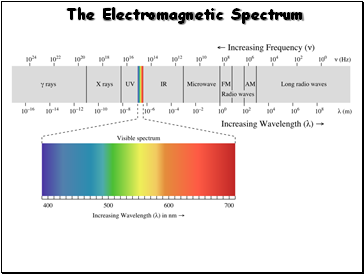
The Electromagnetic Spectrum
Slide 10
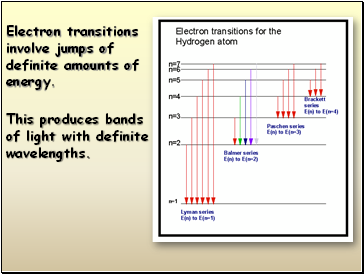
This produces bands
of light with definite wavelengths.
Electron transitions involve jumps of definite amounts of energy.
Slide 11
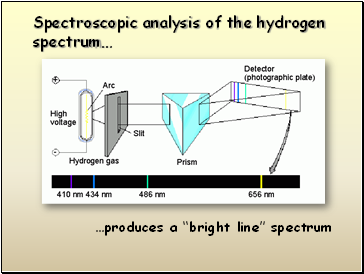
…produces a “bright line” spectrum
1 2
Contents
- The Dilemma of the Atom
- Wave-Particle Duality
- The Wave-like Electron
- Answering the Dilemma of the Atom
- The Electromagnetic Spectrum
- Flame Tests
- Exclusion Notice
- Electron Energy in Hydrogen
Last added presentations
- Solar Thermal Energy
- Magnetic field uses sound waves to ignite sun's ring of fire
- Thermal Energy
- Health Physics
- Practical Applications of Solar Energy
- Newton’s Law of Gravity
- Newton’s laws of motion