Arrhenius Acids and BasesPage
2
2
A. HBr (aq) 1. bromic acid
2. bromous acid
3. hydrobromic acid
B. H2CO3 1. carbonic acid
2. hydrocarbonic acid
3. carbonous acid
Slide 11
LecturePLUS Timberlake
11
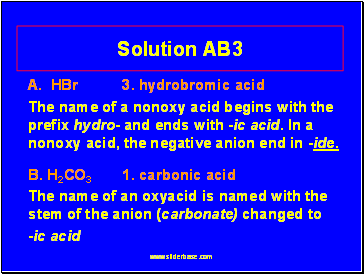
Solution AB3
A. HBr 3. hydrobromic acid
The name of a nonoxy acid begins with the prefix hydro- and ends with -ic acid. In a nonoxy acid, the negative anion end in -ide.
B. H2CO3 1. carbonic acid
The name of an oxyacid is named with the stem of the anion (carbonate) changed to
-ic acid
Slide 12
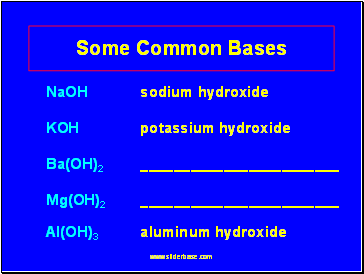
Some Common Bases
LecturePLUS Timberlake
12
NaOH sodium hydroxide
KOH potassium hydroxide
Ba(OH)2
Mg(OH)2 Al(OH)3 aluminum hydroxide
Slide 13
LecturePLUS Timberlake
13
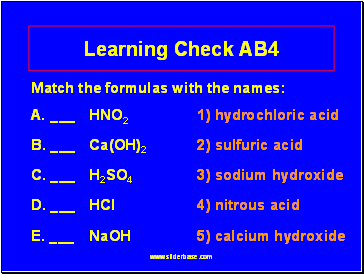
Learning Check AB4
Match the formulas with the names:
A. _ HNO2 1) hydrochloric acid
B. _ Ca(OH)2 2) sulfuric acid
C. _ H2SO4 3) sodium hydroxide
D. _ HCl 4) nitrous acid
E. _ NaOH 5) calcium hydroxide
Slide 14
LecturePLUS Timberlake
14
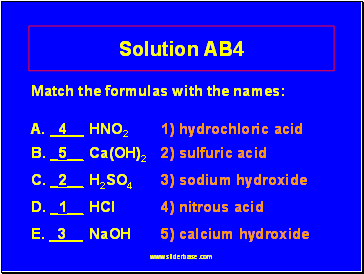
Solution AB4
Match the formulas with the names:
A. _4 HNO2 1) hydrochloric acid
B. _5 Ca(OH)2 2) sulfuric acid
C. _2 H2SO4 3) sodium hydroxide
D. _1 HCl 4) nitrous acid
E. _3 NaOH 5) calcium hydroxide
Slide 15
LecturePLUS Timberlake
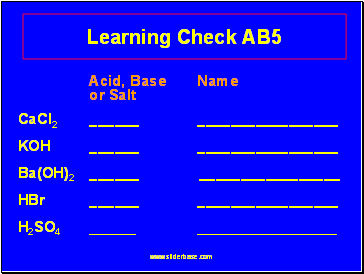
15 Learning Check AB5
Acid, Base Name
or Salt
CaCl2 _
KOH _
Ba(OH)2 _
HBr _
H2SO4
Slide 16
LecturePLUS Timberlake
16
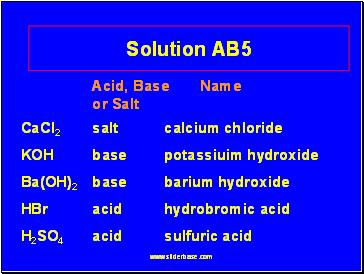
Solution AB5
Acid, Base Name
or Salt
CaCl2 salt calcium chloride
KOH base potassiuim hydroxide
Ba(OH)2 base barium hydroxide
HBr acid hydrobromic acid
H2SO4 acid sulfuric acid
Slide 17
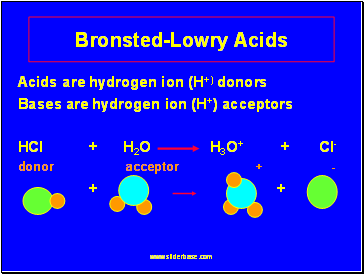
Bronsted-Lowry Acids
LecturePLUS Timberlake
17
Acids are hydrogen ion (H+) donors
Bases are hydrogen ion (H+) acceptors
HCl + H2O H3O+ + Cl-
donor acceptor + -
+ +
1 2
Contents
Last added presentations
- Practical Applications of Solar Energy
- Magnetic field uses sound waves to ignite sun's ring of fire
- Friction
- Radiation Safety and Operations
- Space Radiation
- Soil and Plant Nutrition
- Solar Thermal Energy
