Acids and Bases. Experimental DefinitionsPage
2
2
Strong and Weak Acids and Bases
Strong bases ionize completely in water solution. 100%
NaOH(aq) --> Na+(aq) + OH-(aq)
Weak bases only partially ionize in water solution.
NH3(aq) + H2O --> NH4+(aq) + OH-(aq)
Slide 13
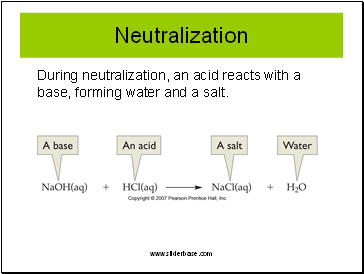
Neutralization
During neutralization, an acid reacts with a base, forming water and a salt.
Slide 14
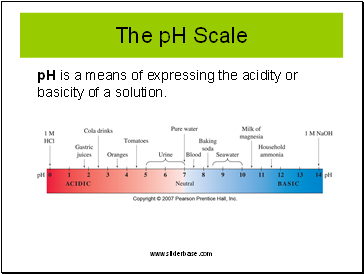
The pH Scale
pH is a means of expressing the acidity or basicity of a solution.
Slide 15
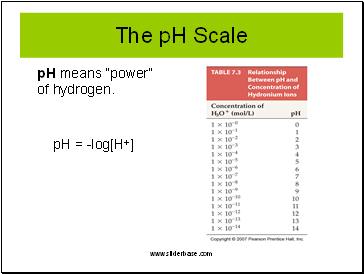
The pH Scale
pH means “power” of hydrogen.
pH = -log[H+]
Slide 16
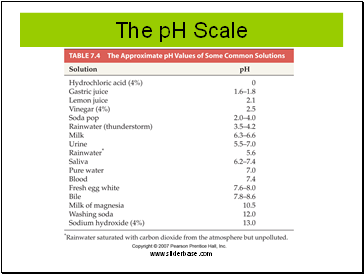
The pH Scale
Slide 17
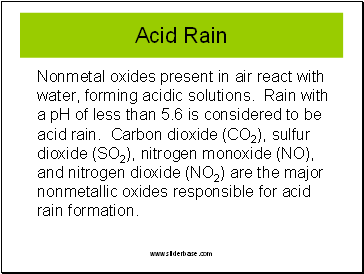
Acid Rain
Nonmetal oxides present in air react with water, forming acidic solutions. Rain with a pH of less than 5.6 is considered to be acid rain. Carbon dioxide (CO2), sulfur dioxide (SO2), nitrogen monoxide (NO), and nitrogen dioxide (NO2) are the major nonmetallic oxides responsible for acid rain formation.
Slide 18
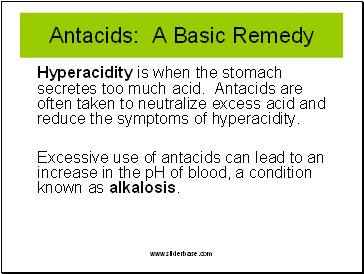
Antacids: A Basic Remedy
Hyperacidity is when the stomach secretes too much acid. Antacids are often taken to neutralize excess acid and reduce the symptoms of hyperacidity.
Excessive use of antacids can lead to an increase in the pH of blood, a condition known as alkalosis.
Slide 19
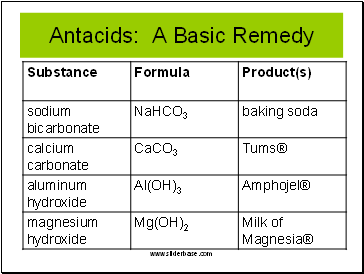
Antacids: A Basic Remedy
Slide 20
Antacids: A Basic Remedy
Slide 21
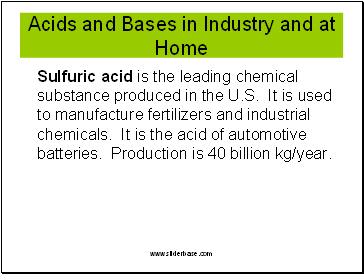
Acids and Bases in Industry and at Home
Sulfuric acid is the leading chemical substance produced in the U.S. It is used to manufacture fertilizers and industrial chemicals. It is the acid of automotive batteries. Production is 40 billion kg/year.
Slide 22
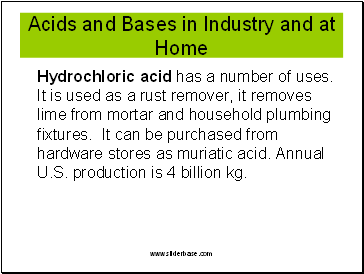
Acids and Bases in Industry and at Home
Hydrochloric acid has a number of uses. It is used as a rust remover, it removes lime from mortar and household plumbing fixtures. It can be purchased from hardware stores as muriatic acid. Annual U.S. production is 4 billion kg.
Slide 23
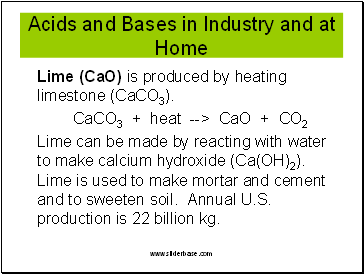
Acids and Bases in Industry and at Home
Lime (CaO) is produced by heating limestone (CaCO3).
CaCO3 + heat --> CaO + CO2
Contents
- Acids and Bases: Experimental Definitions
- Acids and Bases
- Acids, Bases, and Salts
- Strong and Weak Acids and Bases
- Neutralization
- The pH Scale
- Acid Rain
- Antacids: A Basic Remedy
- Acids and Bases in Industry and at Home
- Acids and Bases in Health and Disease
Last added presentations
- Health Physics
- Buoyancy
- Newton’s third law of motion
- Radiation Safety and Operations
- Upcoming Classes
- Simulation at NASA for the Space Radiation Effort
- Motion