Balancing Chemical EquationsPage
1
1
Slide 1
Balancing Chemical Equations
Slide 2
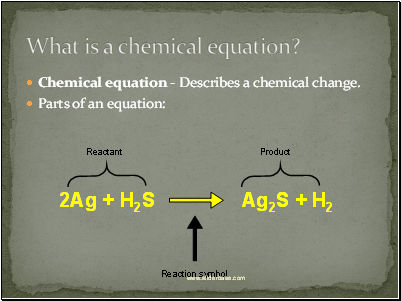
What is a chemical equation?
Chemical equation - Describes a chemical change.
Parts of an equation:
Slide 3
Reactants and Products
Reactant - The chemical(s) you start with before the reaction.
Written on left side of equation.
Product - The new chemical(s) formed by the reaction.
Right side of equation.
Slide 4
Subscripts and Coefficients
Subscript - shows how many atoms of an element are in a molecule.
EX: H2O
2 atoms of hydrogen (H)þ
1 atom of oxygen (O)þ
Coefficient - shows how many molecules there are of a particular chemical.
EX: 3 H2O
Means there are 3 water molecules.
Slide 5
2H2 + O2 2H2O
A Chemical Reaction
Slide 6
Law of Conservation of Mass
In a chem. rxn, matter is neither created nor destroyed.
In other words, the number and type of atoms going INTO a rxn must be the same as the number and type of atoms coming OUT.
If an equation obeys the Law of Conservation, it is balanced.
Slide 7
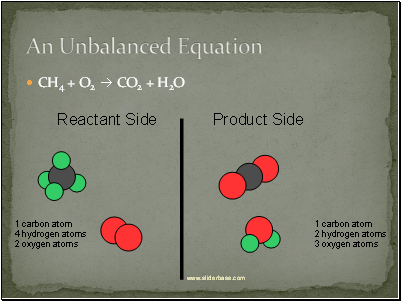
An Unbalanced Equation
CH4 + O2 CO2 + H2O
Reactant Side
Product Side
1 carbon atom
4 hydrogen atoms
2 oxygen atoms
1 carbon atom
2 hydrogen atoms
3 oxygen atoms
Slide 8
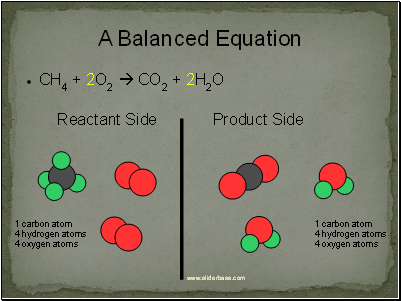
A Balanced Equation
CH4 + 2O2 CO2 + 2H2O
Reactant Side
Product Side
1 carbon atom
4 hydrogen atoms
4 oxygen atoms
1 carbon atom
4 hydrogen atoms
4 oxygen atoms
Slide 9
Rules of the Game
1. Matter cannot be created or destroyed.
2. Subscripts cannot be added, removed, or changed.
3. You can only change coefficients.
4. Coefficients can only go in front of chem. formulas .NEVER in the middle of a formula.
A few extra tips:
Try balancing big formulas first; save free elements for last.
If the same polyatomic ion appears on both sides of the equation, it’s usually okay to treat it as one unit.
There is no one particular way to balance equations. Some equations are harder to balance than others and might require some creativity to solve.
Slide 10
1 2
Contents
- What is a chemical equation?
- Reactants and Products
- Subscripts and Coefficients
- Law of Conservation of Mass
- An Unbalanced Equation
- A Balanced Equation
- Rules of the Game
- Balancing Equations
Last added presentations
- Understanding Heat Transfer, Conduction, Convection and Radiation
- The Effects of Radiation on Living Things
- Newton's Laws
- Health Physics
- Practical Applications of Solar Energy
- Buoyancy
- Newton's laws of motion