PH Scale and CalculationsPage
2
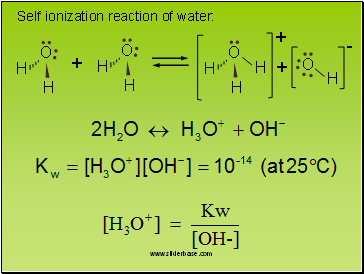
2
O
H
H
O
H
H
O
Self ionization reaction of water:
+
H
+
+
-
Slide 10
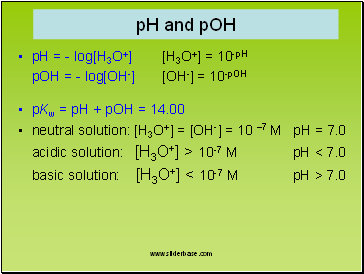
pH and pOH
pH = - log[H3O+] [H3O+] = 10-pH
pOH = - log[OH-] [OH-] = 10-pOH
pKw = pH + pOH = 14.00
neutral solution: [H3O+] = [OH-] = 10 –7 M pH = 7.0
acidic solution: [H3O+] > 10-7 M pH < 7.0
basic solution: [H3O+] < 10-7 M pH > 7.0
Slide 11
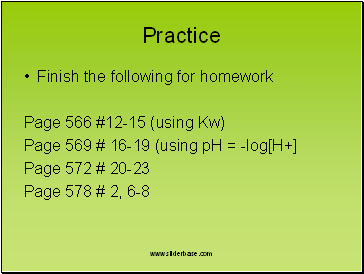
Practice
Finish the following for homework
Page 566 #12-15 (using Kw)
Page 569 # 16-19 (using pH = -log[H+]
Page 572 # 20-23
Page 578 # 2, 6-8
Go to page:
1 2
1 2
Contents
Last added presentations
- Newton's Laws
- Newton’s law of universal gravitation
- Simulation at NASA for the Space Radiation Effort
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Gravitation
- Waves & Sound
- Sound
© 2010-2025 powerpoint presentations