The ATOMPage
2
2
He received the Nobel Prize in physics in 1922 for his theory.
Slide 14
Quantum Mechanics Model
Slide 15
Quantum Mechanics Model
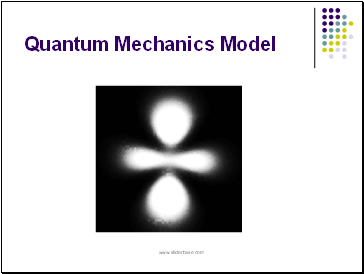
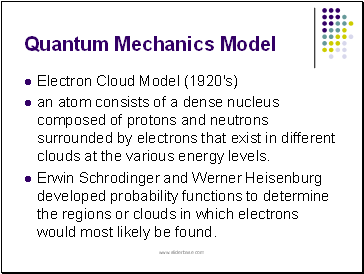
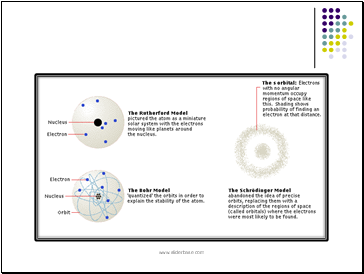
Electron Cloud Model (1920's)
an atom consists of a dense nucleus composed of protons and neutrons surrounded by electrons that exist in different clouds at the various energy levels.
Erwin Schrodinger and Werner Heisenburg developed probability functions to determine the regions or clouds in which electrons would most likely be found.
Slide 16
Slide 17
A combination of all models…
Slide 18
Drawing Bohr Diagrams
Atomic number:
identifies the element
equal to the number of protons in the nucleus
since atoms are electrically neutral, # of protons = # of electrons
Mass number:
# of protons + # of neutrons
# neutrons = atomic mass (rounded off) – atomic #
Protons and neutrons account for most of the mass of the atom
Slide 19
Bohr Diagrams (continued)
an energy level represents a specific value of energy of an electron and corresponds to a general location around the nucleus
the number of occupied energy levels in any atom is normally the same as the period number in which an atom appears
Slide 20
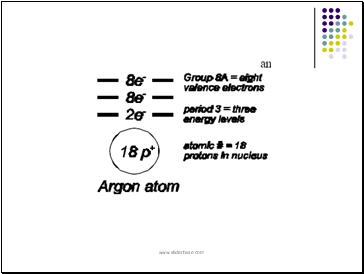
for the first 3 energy levels, the maximum number of electrons that can be present are 2, 8 and 8 in order of increasing energy (increasing distance from the nucleus)
a lower energy level is filled with electrons to its maximum level before the next level is started.
The electrons in the highest (outermost) occupied energy level are called valence electrons. Number of valence electrons is the same as the group number for group A elements (1,2,13-18).
Slide 21
Bohr Diagrams (continued)
Draw a circle for the nucleus
Put in the number of protons and neutrons inside the circle.
Determine the number of electrons and place them in energy levels starting closest to the nucleus and filling them in the order of 2, 8, 8.
Slide 22
Slide 23
Stable Atoms
have low chemical reactivity
include noble gases, all of which have 8 valence electrons (except He, which has 2)
Contents
- Atoms
- Models of the Atom - History
- John Dalton (1803)
- J. J. Thomson (1897)
- Ernest Rutherford (1908) - Nuclear
- Neils Bohr (1913)
- Quantum Mechanics Model
- Drawing Bohr Diagrams
- Stable Atoms
- Ions
- Sodium
- Chlorine
- How ionic bonds form:
Last added presentations
- Simulation at NASA for the Space Radiation Effort
- Geophysical Concepts, Applications and Limitations
- Mechanics Lecture
- Buoyancy
- Resource Acquisition and Transport in Vascular Plants
- Understanding Heat Transfer, Conduction, Convection and Radiation
- The Effects of Radiation on Living Things