The ATOMPage
1
1
Slide 1
The ATOM
Slide 2
Slide 3
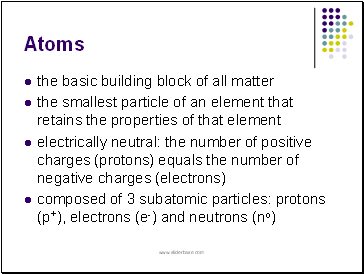
Atoms
the basic building block of all matter
the smallest particle of an element that retains the properties of that element
electrically neutral: the number of positive charges (protons) equals the number of negative charges (electrons)
composed of 3 subatomic particles: protons (p+), electrons (e-) and neutrons (no)
Slide 4
Models of the Atom - History
Democritus a fifth century B.C. Greek philosopher proposed that all matter was composed of indivisible particles called atoms (“atoma” - Greek for indivisible).
Slide 5
Slide 6
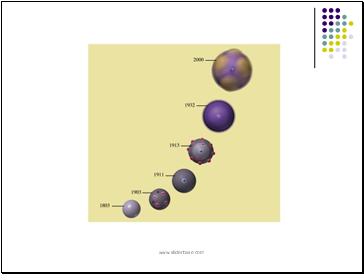
John Dalton (1803)- Billiard Ball
Slide 7
John Dalton (1803)
Billiard Ball Model
viewed the atom as a small solid sphere. He really got the "ball" rolling for modern chemistry!
Each element was composed of the same kind of atoms.
Compounds are composed of atoms in specific ratios.
Chemical reactions are rearrangements of atoms (mass is conserved).
Slide 8
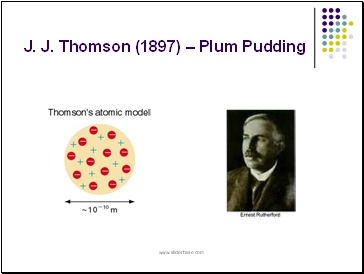
J. J. Thomson (1897) – Plum Pudding
Slide 9
J. J. Thomson (1897)
Plum Pudding Model
proposed that the atom was a sphere of positive electricity with negative particles imbedded throughout after discovering the electron
a discovery for which he was awarded the Nobel Prize in physics in 1906.
Slide 10
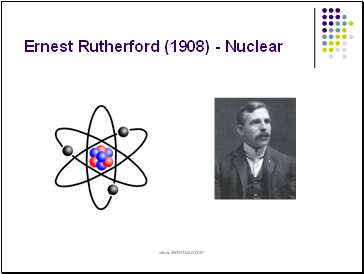
Ernest Rutherford (1908) - Nuclear
Slide 11
Ernest Rutherford (1908)
discovered that the atom is mostly empty space with a dense positively charged nucleus surrounded by negative electrons.
Rutherford received the Nobel Prize in chemistry in 1908 for his contributions into the structure of the atom.
Slide 12
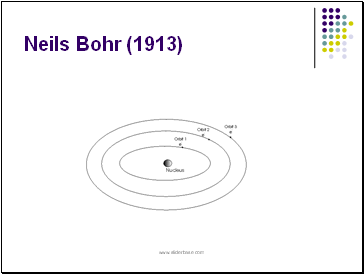
Neils Bohr (1913)
Slide 13
Neils Bohr (1913)
proposed that electrons traveled in circular orbits and that only certain orbits were allowed.
Contents
- Atoms
- Models of the Atom - History
- John Dalton (1803)
- J. J. Thomson (1897)
- Ernest Rutherford (1908) - Nuclear
- Neils Bohr (1913)
- Quantum Mechanics Model
- Drawing Bohr Diagrams
- Stable Atoms
- Ions
- Sodium
- Chlorine
- How ionic bonds form:
Last added presentations
- Radiation Safety and Operations
- Magnetic field uses sound waves to ignite sun's ring of fire
- The Effects of Radiation on Living Things
- Newton’s Laws of Motion
- Gravitation
- History of Modern Astronomy
- Ch 9 Nuclear Radiation