The pH ScalePage
2
2
4.0 x 10-5
Enter 1.0 EE +/- 14 4.0 EE +/- 5
= 2.5 x 10 -10
Slide 11
LecturePLUS Timberlake
11
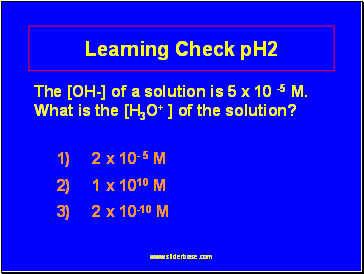
Learning Check pH2
The [OH-] of a solution is 5 x 10 -5 M. What is the [H3O+ ] of the solution?
1) 2 x 10- 5 M
2) 1 x 1010 M
3) 2 x 10-10 M
Slide 12
LecturePLUS Timberlake
12
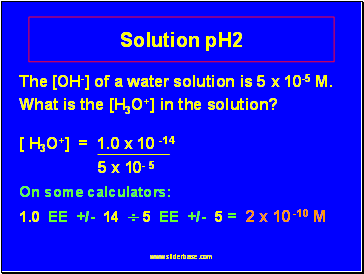
Solution pH2
The [OH-] of a water solution is 5 x 10-5 M. What is the [H3O+] in the solution?
[ H3O+] = 1.0 x 10 -14
5 x 10- 5
On some calculators:
1.0 EE +/- 14 5 EE +/- 5 = 2 x 10 -10 M
Slide 13
LecturePLUS Timberlake
13
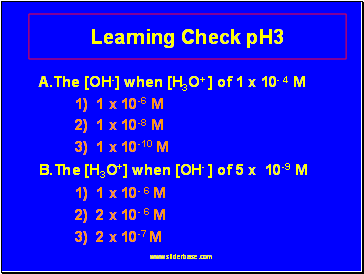
Learning Check pH3
A.The [OH-] when [H3O+ ] of 1 x 10- 4 M
1) 1 x 10-6 M
2) 1 x 10-8 M
3) 1 x 10-10 M
B.The [H3O+] when [OH- ] of 5 x 10-9 M
1) 1 x 10- 6 M
2) 2 x 10- 6 M
3) 2 x 10-7 M
Slide 14
LecturePLUS Timberlake
14
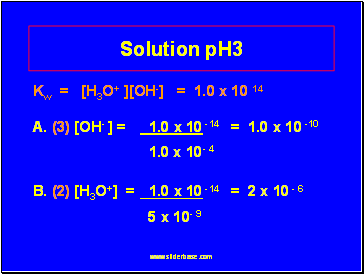
Solution pH3
Kw = [H3O+ ][OH-] = 1.0 x 10 14
A. (3) [OH- ] = 1.0 x 10 -14 = 1.0 x 10 -10
1.0 x 10- 4
B. (2) [H3O+] = 1.0 x 10 -14 = 2 x 10 - 6
5 x 10- 9
Slide 15
pH
LecturePLUS Timberlake
15
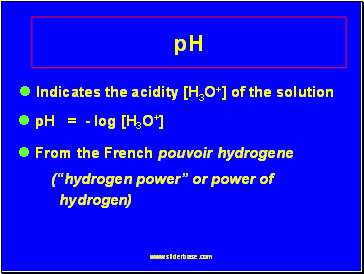
Indicates the acidity [H3O+] of the solution
pH = - log [H3O+]
From the French pouvoir hydrogene
(“hydrogen power” or power of
hydrogen)
Slide 16
LecturePLUS Timberlake
16
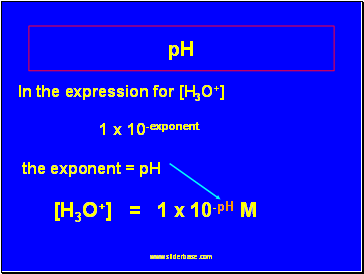
In the expression for [H3O+]
1 x 10-exponent
the exponent = pH
[H3O+] = 1 x 10-pH M
pH
Slide 17
LecturePLUS Timberlake
17
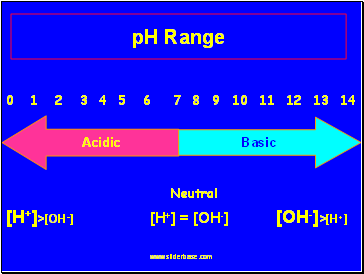
pH Range
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
Neutral
[H+]>[OH-] [H+] = [OH-] [OH-]>[H+]
Acidic
Basic
Slide 18
LecturePLUS Timberlake
18
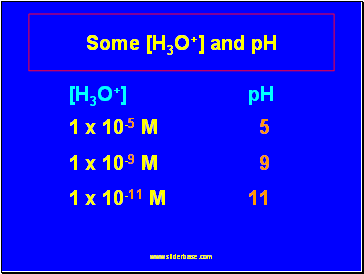
Some [H3O+] and pH
[H3O+] pH
1 x 10-5 M 5
1 x 10-9 M 9
1 x 10-11 M 11
Slide 19
LecturePLUS Timberlake
19
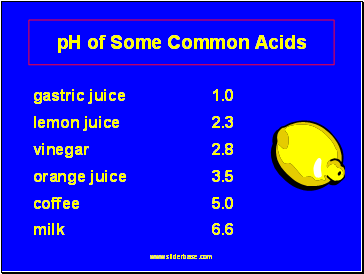
pH of Some Common Acids
gastric juice 1.0
lemon juice 2.3
vinegar 2.8
orange juice 3.5
coffee 5.0
milk 6.6
Slide 20
LecturePLUS Timberlake
20
pH of Some Common Bases
blood 7.4
tears 7.4
seawater 8.4
milk of magnesia 10.6
household ammonia 11.0
Contents
- Ionization of Water
- Pure Water is Neutral
- Ion Product of Water Kw
- Acids
- Bases
- Using the Calculator
- Acid Rain
- Sources of Acid Rain
- Effects of Acid Rain
Last added presentations
- Radiation Safety and Operations
- Solar Thermal Energy
- Space Radiation
- Static and Kinetic Friction
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Newton’s Laws of Motion
- Radioactivity and Nuclear Reactions