Ionic BondingPage
1
1
Slide 1
Ionic Bonding
Slide 2
CA Standards
Students know atoms combine to form molecules by sharing electrons to form covalent or metallic bonds or by exchanging electrons to form ionic bonds.
Students know salt crystals, such as NaCl, are repeating patterns of positive and negative ions held together by electrostatic attraction.
Slide 3
Bonds
Forces that hold groups of atoms
together and make them function
as a unit.
Ionic bonds – transfer of electrons
Covalent bonds – sharing of electrons
Slide 4
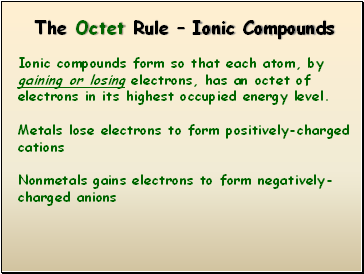
The Octet Rule – Ionic Compounds
Ionic compounds form so that each atom, by gaining or losing electrons, has an octet of electrons in its highest occupied energy level.
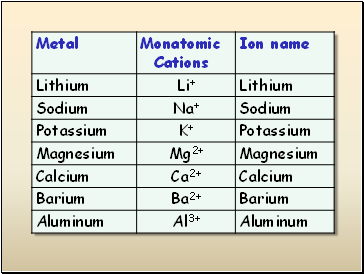
Metals lose electrons to form positively-charged cations
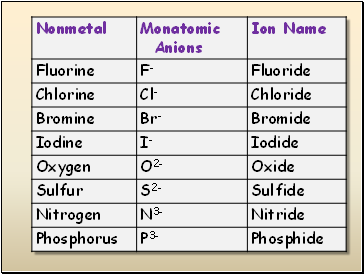
Nonmetals gains electrons to form negatively-charged anions
Slide 5
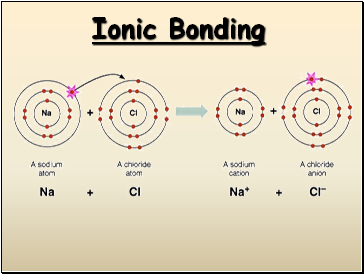
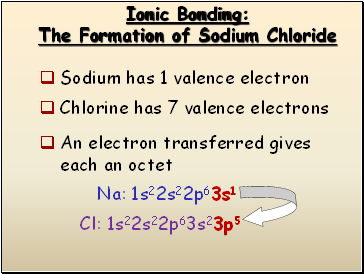
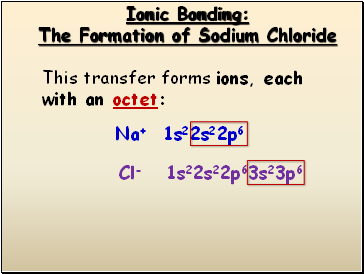
Ionic Bonding
The Formation of Sodium Chloride
Sodium has 1 valence electron
Cl: 1s22s22p63s23p5
Na: 1s22s22p63s1
Chlorine has 7 valence electrons
An electron transferred gives
each an octet
Slide 6
Ionic Bonding: The Formation of Sodium Chloride
Cl- 1s22s22p63s23p6
Na+ 1s22s22p6
This transfer forms ions, each with an octet:
Slide 7
Ionic Bonding: The Formation of Sodium Chloride
Cl-
Na+
The resulting ions come together due to electrostatic attraction
(opposites attract):
The net charge on the compound must equal zero
Slide 8
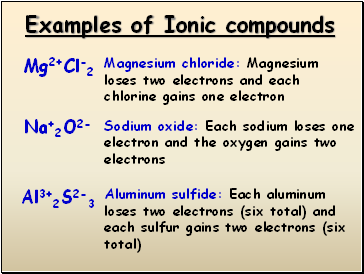
Examples of Ionic compounds
Mg2+Cl-2
Na+2O2-
Magnesium chloride: Magnesium loses two electrons and each chlorine gains one electron
Sodium oxide: Each sodium loses one electron and the oxygen gains two electrons
Al3+2S2-3
Aluminum sulfide: Each aluminum loses two electrons (six total) and each sulfur gains two electrons (six total)
Slide 9
Slide 10
Slide 11
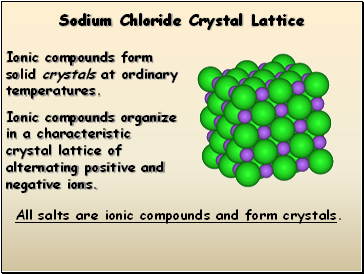
Sodium Chloride Crystal Lattice
Ionic compounds form solid crystals at ordinary temperatures.
1 2
Contents
- Ionic Bonding
- Bonds
- The Octet Rule – Ionic Compounds
- Ionic Bonding
- Examples of Ionic compounds
- Sodium Chloride Crystal Lattice
Last added presentations
- Newton’s Laws of Motion
- Newton’s third law of motion
- Upcoming Classes
- Heat-Energy on the Move
- Geophysical Concepts, Applications and Limitations
- Health Physics
- Newton’s law of universal gravitation