Measurement of Pressure and TemperaturePage
1
1
Slide 1
Pressure and Temperature
William Thomson
“Lord Kelvin”
Slide 2
CA Standards
Slide 3
Measuring Pressure
The first device for measuring atmospheric
pressure was developed by Evangelista Torricelli
during the 17th century.
The device was called a “barometer”
Baro = weight
Meter = measure
Slide 4
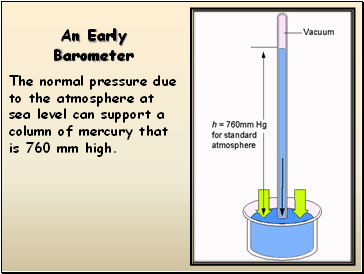
An Early Barometer
The normal pressure due to the atmosphere at sea level can support a column of mercury that is 760 mm high.
Slide 5
Pressure
Is caused by the collisions of molecules with the walls of a container
is equal to force/unit area
Slide 6
Standard Pressure
1 standard atmosphere (atm)
101.3 kPa (kilopascals)
14.7 lbs/in2
760 mm Hg (millimeters of mercury)
760 torr
Slide 7
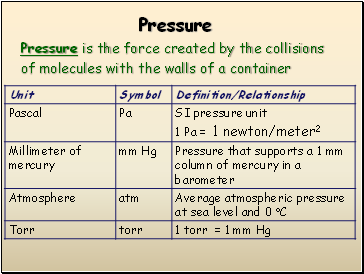
Pressure
Pressure is the force created by the collisions
of molecules with the walls of a container
Slide 8
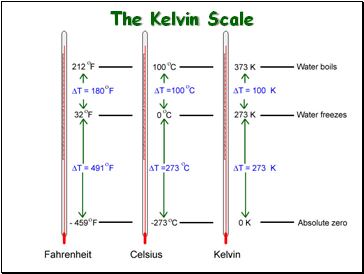
The Kelvin Scale
Slide 9
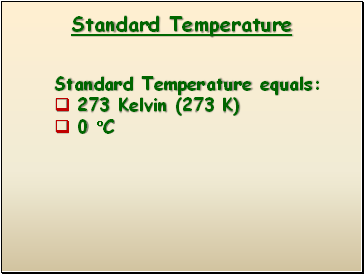
Standard Temperature
Standard Temperature equals:
273 Kelvin (273 K)
0 C
Slide 10
Converting Celsius to Kelvin
Gas law problems involving temperature require that the temperature be in KELVINS!
Kelvins = C + 273
°C = Kelvins - 273
Slide 11
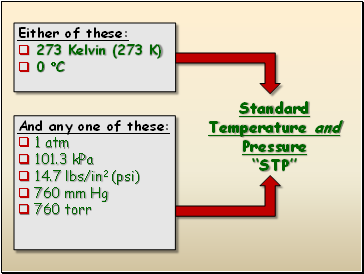
Standard Temperature and Pressure “STP”
Either of these:
273 Kelvin (273 K)
0 C
And any one of these:
1 atm
101.3 kPa
14.7 lbs/in2 (psi)
760 mm Hg
760 torr
Slide 12
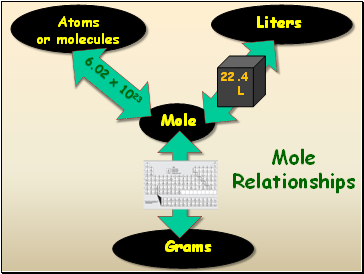
Standard Molar Volume
One mole of a gas at STP has a volume of 22.4 Liters
Slide 13
Mole Relationships
Mole
Atoms
or molecules
Liters
Grams
6.02 x 1023
Atomic
Mass
22. 4 L
22.4
L
Contents
- Measuring Pressure
- An Early Barometer
- Pressure
- Standard Pressure
- The Kelvin Scale
- Standard Temperature
- Converting Celsius to Kelvin
- Standard Molar Volume
Last added presentations
- Ch 9 Nuclear Radiation
- Soil and Plant Nutrition
- Health Physics
- Madame Marie Curie
- Radiation
- Motion
- History of Modern Astronomy