Organic and Biological MoleculesPage
1
1
Slide 1
Simple Organic Chemistry
Basic Structure and Nomenclature
Slide 2
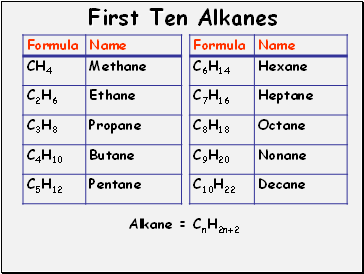
First Ten Alkanes
Alkane = CnH2n+2
Slide 3
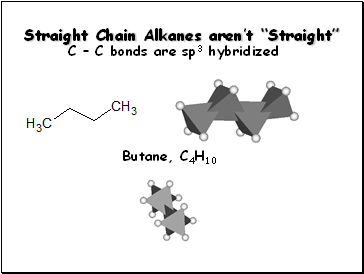
Straight Chain Alkanes aren’t “Straight”
C – C bonds are sp3 hybridized
Butane, C4H10
Slide 4
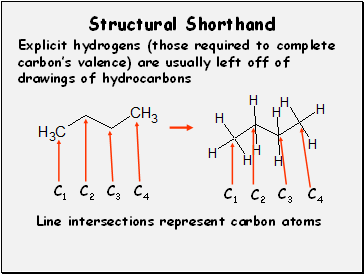
Structural Shorthand
Explicit hydrogens (those required to complete carbon’s valence) are usually left off of drawings of hydrocarbons
Line intersections represent carbon atoms
C1
C1
C2
C2
C3
C3
C4
C4
Slide 5
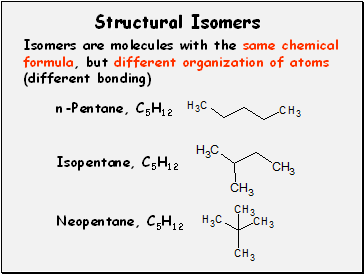
Structural Isomers
Isomers are molecules with the same chemical formula, but different organization of atoms (different bonding)
n-Pentane, C5H12
Isopentane, C5H12
Neopentane, C5H12
Slide 6
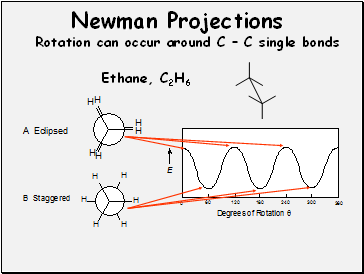
Newman Projections
Ethane, C2H6
Rotation can occur around C – C single bonds
Slide 7
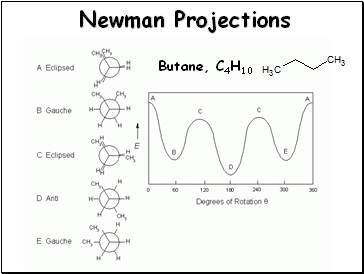
Newman Projections
Butane, C4H10
Slide 8
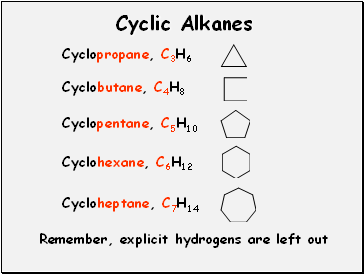
Cyclic Alkanes
Cyclopropane, C3H6
Remember, explicit hydrogens are left out
Cyclobutane, C4H8
Cyclopentane, C5H10
Cyclohexane, C6H12
Cycloheptane, C7H14
Slide 9
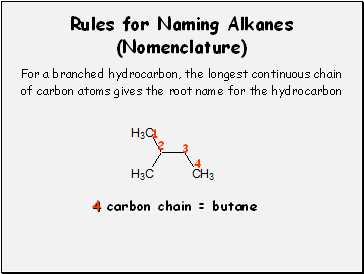
Rules for Naming Alkanes (Nomenclature)
For a branched hydrocarbon, the longest continuous chain
of carbon atoms gives the root name for the hydrocarbon
1
2
3
4
4 carbon chain = butane
Slide 10
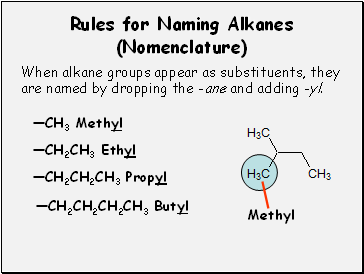
Rules for Naming Alkanes (Nomenclature)
When alkane groups appear as substituents, they
are named by dropping the -ane and adding -yl.
—CH3 Methyl
—CH2CH3 Ethyl
—CH2CH2CH3 Propyl
—CH2CH2CH2CH3 Butyl
Methyl
Slide 11
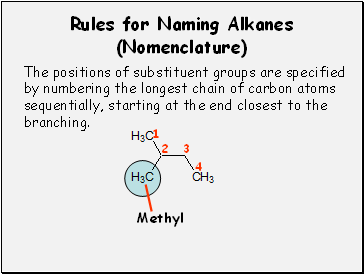
Rules for Naming Alkanes (Nomenclature)
The positions of substituent groups are specified
by numbering the longest chain of carbon atoms
sequentially, starting at the end closest to the
branching.
Methyl
1
2
3
4
Slide 12
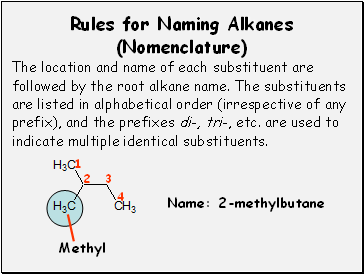
Rules for Naming Alkanes (Nomenclature)
The location and name of each substituent are
Contents
- First Ten Alkanes
- Straight Chain Alkanes aren’t “Straight”
- Structural Shorthand
- Structural Isomers
- Newman Projections
- Cyclic Alkanes
- Rules for Naming Alkanes (Nomenclature)
- Nomenclature Practice
- Alkenes Contain Carbon-Carbon
- Reactions of Alkenes and Alkynes
- Aromatic Hydrocarbons
- Geometric Isomerism in Aromatics
Last added presentations
- Static and Kinetic Friction
- Ch 9 Nuclear Radiation
- Radiation
- Simulation at NASA for the Space Radiation Effort
- Newton’s Law of Gravity
- Friction
- Solar Energy