Organic and Biological MoleculesPage
2
2
followed by the root alkane name. The substituents
are listed in alphabetical order (irrespective of any
prefix), and the prefixes di-, tri-, etc. are used to
indicate multiple identical substituents.
Methyl
1
2
3
4
Name:
2-methylbutane
Slide 13
Nomenclature Practice
Name this compound
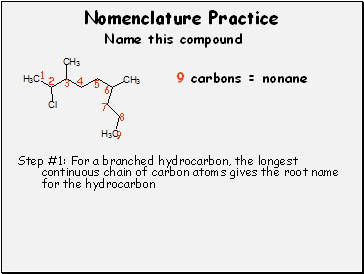
Step #1: For a branched hydrocarbon, the longest continuous chain of carbon atoms gives the root name for the hydrocarbon
1
5
2
4
3
9
6
8
7
9 carbons = nonane
Slide 14
Nomenclature Practice
Name this compound
1
5
2
4
3
9
6
8
7
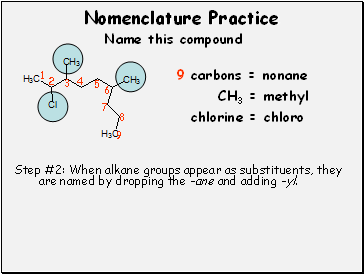
9 carbons = nonane
Step #2: When alkane groups appear as substituents, they are named by dropping the -ane and adding -yl.
CH3 = methyl
chlorine = chloro
Slide 15
Nomenclature Practice
Name this compound
1
5
2
4
3
9
6
8
7
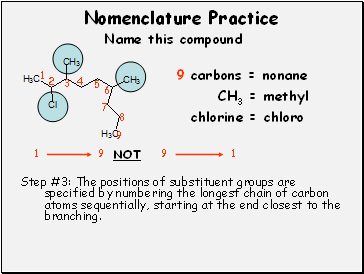
9 carbons = nonane
CH3 = methyl
chlorine = chloro
Step #3: The positions of substituent groups are specified by numbering the longest chain of carbon atoms sequentially, starting at the end closest to the branching.
1
9
NOT
9
1
Slide 16
Nomenclature Practice
Name this compound
1
5
2
4
3
9
6
8
7
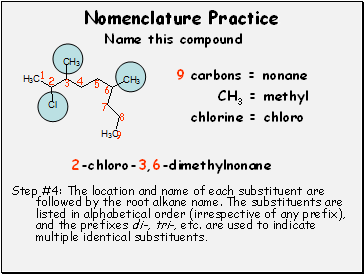
9 carbons = nonane
CH3 = methyl
chlorine = chloro
Step #4: The location and name of each substituent are followed by the root alkane name. The substituents are listed in alphabetical order (irrespective of any prefix), and the prefixes di-, tri-, etc. are used to indicate multiple identical substituents.
2-chloro-3,6-dimethylnonane
Slide 17
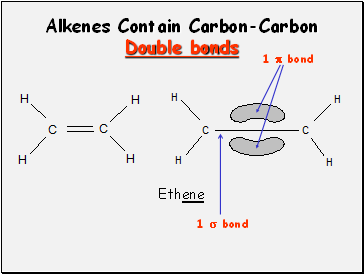
Alkenes Contain Carbon-Carbon
Double bonds
Ethene
1 bond
1 bond
Slide 18
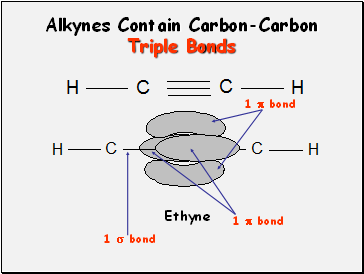
Alkynes Contain Carbon-Carbon Triple Bonds
Ethyne
1 bond
1 bond
1 bond
Slide 19
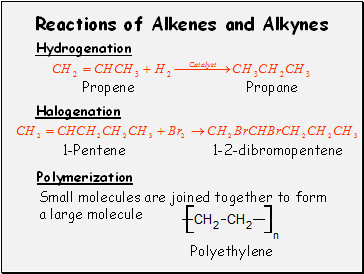
Reactions of Alkenes and Alkynes
Hydrogenation
Propene
Propane
Halogenation
1-Pentene
1-2-dibromopentene
Polymerization
Small molecules are joined together to form a large molecule
Polyethylene
Slide 20
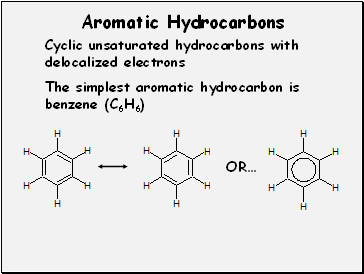
Aromatic Hydrocarbons
Cyclic unsaturated hydrocarbons with
delocalized electrons
Contents
- First Ten Alkanes
- Straight Chain Alkanes aren’t “Straight”
- Structural Shorthand
- Structural Isomers
- Newman Projections
- Cyclic Alkanes
- Rules for Naming Alkanes (Nomenclature)
- Nomenclature Practice
- Alkenes Contain Carbon-Carbon
- Reactions of Alkenes and Alkynes
- Aromatic Hydrocarbons
- Geometric Isomerism in Aromatics
Last added presentations
- Madame Marie Curie
- Simulation at NASA for the Space Radiation Effort
- Heat-Energy on the Move
- Direct heat utilization of geothermal energy
- Newton's Laws
- Health Physics
- Radiation Safety and Operations